Molecular mechanisms of the diabetogenic effects of arsenic: inhibition of insulin signaling by arsenite and methylarsonous acid
- PMID: 17520061
- PMCID: PMC1867998
- DOI: 10.1289/ehp.9867
Molecular mechanisms of the diabetogenic effects of arsenic: inhibition of insulin signaling by arsenite and methylarsonous acid
Abstract
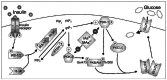
Background: Increased prevalences of diabetes mellitus have been reported among individuals chronically exposed to inorganic arsenic (iAs). However, the mechanisms underlying the diabetogenic effects of iAs have not been characterized. We have previously shown that trivalent metabolites of iAs, arsenite (iAs(III)) and methylarsonous acid (MAs(III)) inhibit insulin-stimulated glucose uptake (ISGU) in 3T3-L1 adipocytes by suppressing the insulin-dependent phosphorylation of protein kinase B (PKB/Akt).
Objectives: Our goal was to identify the molecular mechanisms responsible for the suppression of PKB/Akt phosphorylation by iAs(III) and MAs(III).
Methods: The effects of iAs(III) and MAs(III) on components of the insulin-activated signal transduction pathway that regulate PKB/Akt phosphorylation were examined in 3T3-L1 adipocytes.
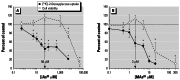
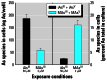
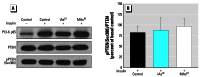
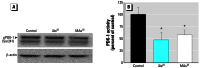
Results: Subtoxic concentrations of iAs(III) or MAs(III) had little or no effect on the activity of phosphatidylinositol 3-kinase (PI-3K), which synthesizes phosphatidylinositol-3,4,5-triphosphate (PIP(3)), or on phosphorylation of PTEN (phosphatase and tensin homolog deleted on chromosome ten), a PIP(3) phosphatase. Neither iAs(III) nor MAs(III) interfered with the phosphorylation of 3-phosphoinositide-dependent kinase-1 (PDK-1) located downstream from PI-3K. However, PDK-1 activity was inhibited by both iAs(III) and MAs(III). Consistent with these findings, PDK-1-catalyzed phosphorylation of PKB/Akt(Thr308) and PKB/Akt activity were suppressed in exposed cells. In addition, PKB/Akt(Ser473) phosphorylation, which is catalyzed by a putative PDK-2, was also suppressed. Notably, expression of constitutively active PKB/Akt restored the normal ISGU pattern in adipocytes treated with either iAs(III) or MAs(III).
Conclusions: These results suggest that inhibition of the PDK-1/PKB/Akt-mediated transduction step is the key mechanism for the inhibition of ISGU in adipocytes exposed to iAs(III) or MAs(III), and possibly for impaired glucose tolerance associated with human exposures to iAs.
Figures





References
-
- Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, et al. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. - PubMed
-
- Altamirano MM, Libreros-Minotta CA, Lara-Lemus R, Calcagno M. Evidence for vicinal thiols and their functional role in glucosamine-6-phosphate deaminase from Escherichia coli. Arch Biochem Biophys. 1989;269:555–561. - PubMed
-
- Bazuine M, Carlotti F, Tafrechi RS, Hoeben RC, Maassen JA. Mitogen-activated protein kinase (MAPK) phosphatase-1 and -4 attenuate p38 MAPK during dexamethasone-induced insulin resistance in 3T3-L1 adipocytes. Mol Endocrinol. 2004;18:1697–1707. - PubMed
-
- Bazuine M, Ouwens DM, Gomes de Mesquita DS, Maassen JA. Arsenite stimulated glucose transport in 3T3-L1 adipocytes involves both Glut4 translocation and p38 MAPK activity. Eur J Biochem. 2003;270:3891–3903. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
