Identification of prediabetes in first-degree relatives at intermediate risk of type I diabetes
- PMID: 17521324
- PMCID: PMC1941942
- DOI: 10.1111/j.1365-2249.2007.03416.x
Identification of prediabetes in first-degree relatives at intermediate risk of type I diabetes
Abstract
Prevention trials of type I diabetes are limited by recruitment of individuals at high risk of the disease. We investigated whether demographic and biological characteristics can identify rapid progressors among first-degree relatives of known patients at intermediate (< 10%) 5-year risk. Diabetes-associated antibodies, random proinsulin : C-peptide (PI/C) ratio and HLA DQ genotype were determined (repeatedly) in 258 islet antibody-positive IA-2Antibody-negative (Abpos/IA-2Aneg) normoglycaemic first-degree relatives. During follow-up (median 81 months), 14 of 258 Abpos/IA-2Aneg relatives developed type I diabetes; 13 (93%) of them had persistent antibodies conferring a 12% [95% confidence interval (CI): 5-19%] 5-year risk of diabetes. In Abpos/IA-2Aneg relatives with persistent antibodies (n = 126), the presence of >/= 1 HLA DQ susceptibility haplotype in the absence of a protective haplotype (P = 0.033) and appearance on follow-up of a high PI/C ratio (P = 0.007) or IA-2A-positivity (P = 0.009) were identified as independent predictors of diabetes. In persistently antibody-positive relatives with HLA DQ risk a recurrently high PI/C ratio or development of IA-2A identified a subgroup (n = 32) comprising 10 of 13 (77%) prediabetic relatives and conferred a 35% (95% CI: 18-53%) 5-year risk. Under age 15 years, 5-year progression (95% CI) was 57% (30-84%) and sensitivity 62%. In the absence of IA-2A, the combination of antibody persistence, HLA DQ risk and elevated PI/C ratio or later development of IA-2A and young age defines a subgroup of relatives with a high risk of type I diabetes (>/= 35% in 5 years). Together with initially IA-2A-positive relatives these individuals qualify for standardized beta cell function tests in view of prevention trials.
Figures
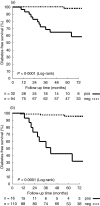
 ) vs. negative (
) vs. negative ( )]. HLA DQ risk haplotypes: HLA DQ2 and HLA DQ8. HLA DQ protective haplotypes: defined in Subjects and methods. Follow-up time started from the time that relatives have entered a particular risk category based on time-dependent markers, i.e. at seroconversion to IA-2A-positivity or at presentation of PI/C ratio > P66 on two different occasions, whichever came first; when positivity for these markers was not achieved, time to event was defined from the second consecutive antibody-positive sample. The individuals still under follow-up in each category (at 12-month intervals) are indicated beneath the time scale.
)]. HLA DQ risk haplotypes: HLA DQ2 and HLA DQ8. HLA DQ protective haplotypes: defined in Subjects and methods. Follow-up time started from the time that relatives have entered a particular risk category based on time-dependent markers, i.e. at seroconversion to IA-2A-positivity or at presentation of PI/C ratio > P66 on two different occasions, whichever came first; when positivity for these markers was not achieved, time to event was defined from the second consecutive antibody-positive sample. The individuals still under follow-up in each category (at 12-month intervals) are indicated beneath the time scale.References
-
- Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–8. - PubMed
-
- Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–608. - PubMed
-
- Chatenoud L. CD3-specific antibody-induced active tolerance: from bench to bedside. Nat Rev Immunol. 2003;3:123–32. - PubMed
-
- Gorus FK, Pipeleers DG the Belgian Diabetes Registry. Prospects for predicting and stopping the development of type 1 diabetes. Best Pract Res Clin Endocrinol Metab. 2001;15:371–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

