The delivery site of a monovalent influenza vaccine within the respiratory tract impacts on the immune response
- PMID: 17521369
- PMCID: PMC2266027
- DOI: 10.1111/j.1365-2567.2007.02641.x
The delivery site of a monovalent influenza vaccine within the respiratory tract impacts on the immune response
Abstract
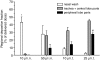
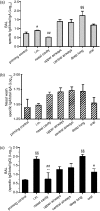
Pulmonary vaccination is a promising immunization route. However, there still remains a crucial need to characterize the different parameters affecting the efficacy of inhaled vaccination. This study aimed at assessing the impact of antigen distribution within the respiratory tract on the immune response to a monovalent A/Panama/2007/99 H3N2 influenza split virus vaccine administered to BALB/c mice. Varying the administration technique allowed the targeting of the vaccine to different sites of the mouse respiratory tract, i.e. the nasal cavity, the upper or central airways, or the deep lung. This targeting was verified by using ovalbumin as a tracer compound. The immune responses generated following influenza vaccine administration to the different respiratory tract sites were compared to each other and to those elicited by intramuscular and peroral intragastric immunization. Delivery of the vaccine to the different respiratory regions generated systemic, local and cellular virus-specific immune responses, which increased with the depth of vaccine deposition, culminating in deep-lung vaccination. The latter induced virus-specific serum immunoglobulin G and neutralizing antibody titres as elevated as intramuscular vaccination, whereas the production of mucosal secretory immunoglobulin A was significantly superior in deep-lung-vaccinated animals. The analysis of cytokines secreted by mononuclear cells during an in vitro recall response indicated that deep-lung vaccination induced a local shift of the cellular immune response towards a T helper type 1 phenotype as compared to intramuscular vaccination. In conclusion, antigen distribution within the respiratory tract has a major effect on the immune response, with the deep lung as the best target for inhaled influenza vaccination.
Figures


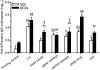
References
-
- Glezen WP. Serious morbidity and mortality associated with influenza epidemics. Epidemiol Rev. 1982;4:25–44. - PubMed
-
- World Health Organization. Influenza Fact Sheet. Geneva: World Health Organization; 2003. no. 211.
-
- Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol. 2004;59:1–15. - PubMed
-
- McElhaney JE. The unmet need in the elderly: designing new influenza vaccines for older adults. Vaccine. 2005;23(Suppl. 1):S10–S25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

