Immune system changes during simulated planetary exploration on Devon Island, high arctic
- PMID: 17521440
- PMCID: PMC1890299
- DOI: 10.1186/1471-2172-8-7
Immune system changes during simulated planetary exploration on Devon Island, high arctic
Abstract
Background: Dysregulation of the immune system has been shown to occur during spaceflight, although the detailed nature of the phenomenon and the clinical risks for exploration class missions have yet to be established. Also, the growing clinical significance of immune system evaluation combined with epidemic infectious disease rates in third world countries provides a strong rationale for the development of field-compatible clinical immunology techniques and equipment. In July 2002 NASA performed a comprehensive immune assessment on field team members participating in the Haughton-Mars Project (HMP) on Devon Island in the high Canadian Arctic. The purpose of the study was to evaluate the effect of mission-associated stressors on the human immune system. To perform the study, the development of techniques for processing immune samples in remote field locations was required. Ten HMP-2002 participants volunteered for the study. A field protocol was developed at NASA-JSC for performing sample collection, blood staining/processing for immunophenotype analysis, whole-blood mitogenic culture for functional assessments and cell-sample preservation on-location at Devon Island. Specific assays included peripheral leukocyte distribution; constitutively activated T cells, intracellular cytokine profiles, plasma cortisol and EBV viral antibody levels. Study timepoints were 30 days prior to mission start, mid-mission and 60 days after mission completion.
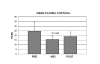
Results: The protocol developed for immune sample processing in remote field locations functioned properly. Samples were processed on Devon Island, and stabilized for subsequent analysis at the Johnson Space Center in Houston. The data indicated that some phenotype, immune function and stress hormone changes occurred in the HMP field participants that were largely distinct from pre-mission baseline and post-mission recovery data. These immune changes appear similar to those observed in astronauts following spaceflight.
Conclusion: The immune system changes described during the HMP field deployment validate the use of the HMP as a ground-based spaceflight/planetary exploration analog for some aspects of human physiology. The sample processing protocol developed for this study may have applications for immune studies in remote terrestrial field locations. Elements of this protocol could possibly be adapted for future in-flight immunology studies conducted during space missions.
Figures





References
-
- Sonnenfeld G, Butel JS, Shearer WT. Effects of the space flight environment on the immune system. Rev Environ Health. 2003;18:1–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

