Zfp423/OAZ participates in a developmental switch during olfactory neurogenesis
- PMID: 17521568
- PMCID: PMC2866517
- DOI: 10.1016/j.neuron.2007.04.029
Zfp423/OAZ participates in a developmental switch during olfactory neurogenesis
Abstract
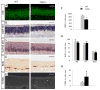
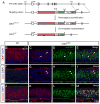
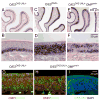
The coordination of gene expression is critical for cell differentiation and the subsequent establishment of tissue function. We show here that a multiple zinc finger transcription factor, Zfp423/OAZ, is transiently expressed in newly differentiating olfactory-receptor neurons (ORNs) and has a key role in coordinating the expression of immature and mature stage-specific genes. OAZ deletion in mice impairs aspects of ORN differentiation, particularly the patterns of axonal projection to the olfactory bulb. OAZ gain-of-function experiments show that sustained OAZ expression throughout ORN maturation arrests ORN development at an immature stage and alters OR gene expression. Importantly, reintroducing OAZ expression in mature ORNs suppresses mature marker expression and reactivates immature-specific markers. Together, these experiments suggest that OAZ participates in a developmental switch regulating the transition from differentiation to maturation in ORNs.
Figures





Similar articles
-
Zfp423/OAZ mutation reveals the importance of Olf/EBF transcription activity in olfactory neuronal maturation.J Neurosci. 2012 Oct 3;32(40):13679-88a. doi: 10.1523/JNEUROSCI.6190-11.2012. J Neurosci. 2012. PMID: 23035080 Free PMC article.
-
The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons.Neuron. 2006 Feb 16;49(4):503-16. doi: 10.1016/j.neuron.2006.01.018. Neuron. 2006. PMID: 16476661
-
Fezl is required for the birth and specification of corticospinal motor neurons.Neuron. 2005 Sep 15;47(6):817-31. doi: 10.1016/j.neuron.2005.08.030. Neuron. 2005. PMID: 16157277
-
Leukemia inhibitory factor inhibits neuronal terminal differentiation through STAT3 activation.Proc Natl Acad Sci U S A. 2002 Jun 25;99(13):9015-20. doi: 10.1073/pnas.132131699. Proc Natl Acad Sci U S A. 2002. PMID: 12084939 Free PMC article.
-
Caspase-3 and caspase-9 mediate developmental apoptosis in the mouse olfactory system.J Comp Neurol. 2004 Jun 14;474(1):136-48. doi: 10.1002/cne.20120. J Comp Neurol. 2004. PMID: 15156583
Cited by
-
Transcriptional regulation of the proto-oncogene Zfp521 by SPI1 (PU.1) and HOXC13.Genesis. 2016 Oct;54(10):519-533. doi: 10.1002/dvg.22963. Epub 2016 Aug 29. Genesis. 2016. PMID: 27506447 Free PMC article.
-
Aberrant ZNF423 impedes B cell differentiation and is linked to adverse outcome of ETV6-RUNX1 negative B precursor acute lymphoblastic leukemia.J Exp Med. 2013 Oct 21;210(11):2289-304. doi: 10.1084/jem.20130497. Epub 2013 Sep 30. J Exp Med. 2013. PMID: 24081948 Free PMC article.
-
Sox5 Is a DNA-binding cofactor for BMP R-Smads that directs target specificity during patterning of the early ectoderm.Dev Cell. 2014 Nov 10;31(3):374-382. doi: 10.1016/j.devcel.2014.10.003. Epub 2014 Nov 10. Dev Cell. 2014. PMID: 25453832 Free PMC article.
-
Differential expression of microRNAs among cell populations in the regenerating adult mouse olfactory epithelium.PLoS One. 2017 Nov 6;12(11):e0187576. doi: 10.1371/journal.pone.0187576. eCollection 2017. PLoS One. 2017. PMID: 29107942 Free PMC article.
-
ZFP423 coordinates Notch and bone morphogenetic protein signaling, selectively up-regulating Hes5 gene expression.J Biol Chem. 2010 Oct 1;285(40):30814-24. doi: 10.1074/jbc.M110.142869. Epub 2010 Jun 14. J Biol Chem. 2010. PMID: 20547764 Free PMC article.
References
-
- Bakalyar HA, Reed RR. Identification of a specialized adenylyl cyclase that may mediate odorant detection. Science. 1990;250:1403–1406. - PubMed
-
- Brunet LJ, Gold GH, Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. 1996;17:681–693. - PubMed
-
- Calof AL, Chikaraishi DM. Analysis of neurogenesis in a mammalian neuroepithelium: proliferation and differentiation of an olfactory neuron precursor in vitro. Neuron. 1989;3:115–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

