A functional heat shock protein 90 chaperone is essential for efficient flock house virus RNA polymerase synthesis in Drosophila cells
- PMID: 17522196
- PMCID: PMC1951356
- DOI: 10.1128/JVI.00189-07
A functional heat shock protein 90 chaperone is essential for efficient flock house virus RNA polymerase synthesis in Drosophila cells
Abstract
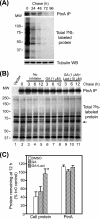
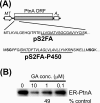
The molecular chaperone heat shock protein 90 (Hsp90) is involved in multiple cellular processes including protein maturation, complex assembly and disassembly, and intracellular transport. We have recently shown that a disruption of Hsp90 activity in cultured Drosophila melanogaster cells suppresses Flock House virus (FHV) replication and the accumulation of protein A, the FHV RNA-dependent RNA polymerase. In the present study, we investigated whether the defect in FHV RNA polymerase accumulation induced by Hsp90 suppression was secondary to an effect on protein A synthesis, degradation, or intracellular membrane association. Treatment with the Hsp90-specific inhibitor geldanamycin selectively reduced FHV RNA polymerase synthesis by 80% in Drosophila S2 cells stably transfected with an inducible protein A expression plasmid. The suppressive effect of geldanamycin on protein A synthesis was not attenuated by proteasome inhibition, nor was it sensitive to changes in either the mRNA untranslated regions or protein A intracellular membrane localization. Furthermore, geldanamycin did not promote premature protein A degradation, nor did it alter the extremely rapid kinetics of protein A membrane association. These results identify a novel role for Hsp90 in facilitating viral RNA polymerase synthesis in Drosophila cells and suggest that FHV subverts normal cellular pathways to assemble functional replication complexes.
Figures



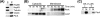

References
-
- Ball, L. A., and K. L. Johnson. 1998. Nodaviruses of insects, p. 225-267. In L. K. Miller and L. A. Ball (ed.), The insect viruses. Plenum Publishing Corporation, New York, NY.
-
- Bangham, A. D., R. W. Horne, A. M. Glauert, J. T. Dingle, and J. A. Lucy. 1962. Action of saponin on biological cell membranes. Nature 196:952-955. - PubMed
-
- Bonifacino, J. S. 2000. Characterization of cellular proteins, p. 5.0.1-5.5.11. In K. S. Morgan (ed.), Current protocols in cell biology. John Wiley & Sons, Inc., New York, NY.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

