Studying hepatitis C virus: making the best of a bad virus
- PMID: 17522203
- PMCID: PMC1951464
- DOI: 10.1128/JVI.00753-07
Studying hepatitis C virus: making the best of a bad virus
Figures
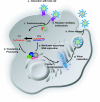
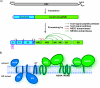
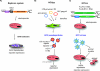

References
-
- Acton, S., A. Rigotti, K. T. Landschulz, S. Xu, H. H. Hobbs, and M. Krieger. 1996. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 271:518-520. - PubMed
-
- Anonymous. 2002. National Institutes of Health Consensus Development Conference statement: management of hepatitis C 2002 (June 10-12, 2002). Gastroenterology 123:2082-2099. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

