Human immunodeficiency virus type 1 assembly, budding, and cell-cell spread in T cells take place in tetraspanin-enriched plasma membrane domains
- PMID: 17522207
- PMCID: PMC1951303
- DOI: 10.1128/JVI.01845-06
Human immunodeficiency virus type 1 assembly, budding, and cell-cell spread in T cells take place in tetraspanin-enriched plasma membrane domains
Abstract
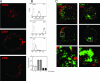
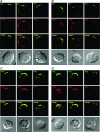
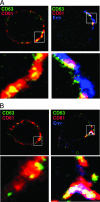
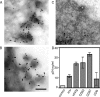
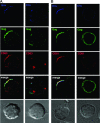
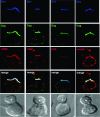
Human immunodeficiency virus type-1 (HIV-1) egress from infected CD4+ T cells is thought to be via assembly and budding at the plasma membrane and may involve components of the T-cell secretory apparatus, including tetraspanins. However, many studies on HIV-1 assembly have examined the trafficking of viral proteins in isolation, and most have used immortalized epithelial, fibroblastic, or hematopoietic cell lines that may not necessarily reflect natural infection of susceptible T cells. Here we have used immunofluorescence and cryoimmunoelectron microscopy (CEM) to examine protein transport during HIV-1 assembly in productively infected Jurkat CD4+ T cells and primary CD4+ T cells. The HIV-1 envelope glycoprotein (Env) and the core protein (Gag) colocalize strongly with CD63 and CD81 and less strongly with CD9, whereas no colocalization was seen between Env or Gag and the late endosome/lysosomal marker Lamp2. CEM revealed incorporation of CD63 and CD81 but not Lamp2 into virions budding at the plasma membrane, and this was supported by immunoprecipitation studies, confirming that HIV-1 egress in T cells is trafficked via tetraspanin-enriched membrane domains (TEMs) that are distinct from lysosomal compartments. CD63, CD81, and, to a lesser extent, CD9 were recruited to the virological synapse (VS), and antibodies against these tetraspanins reduced VS formation. We propose that HIV-1 promotes virus assembly and cell-cell transfer in T cells by targeting plasma membrane TEMs.
Figures

References
-
- Batonick, M., M. Favre, M. Boge, P. Spearman, S. Honing, and M. Thali. 2005. Interaction of HIV-1 Gag with the clathrin-associated adaptor AP-2. Virology 342:190-200. - PubMed
-
- Boge, M., S. Wyss, J. S. Bonifacino, and M. Thali. 1998. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J. Biol. Chem. 273:15773-15778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

