Up-regulation of heat shock proteins is essential for cold survival during insect diapause
- PMID: 17522254
- PMCID: PMC2040864
- DOI: 10.1073/pnas.0703538104
Up-regulation of heat shock proteins is essential for cold survival during insect diapause
Abstract
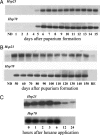
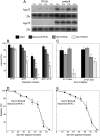
Diapause, the dormancy common to overwintering insects, evokes a unique pattern of gene expression. In the flesh fly, most, but not all, of the fly's heat shock proteins (Hsps) are up-regulated. The diapause up-regulated Hsps include two members of the Hsp70 family, one member of the Hsp60 family (TCP-1), at least four members of the small Hsp family, and a small Hsp pseudogene. Expression of an Hsp70 cognate, Hsc70, is uninfluenced by diapause, and Hsp90 is actually down-regulated during diapause, thus diapause differs from common stress responses that elicit synchronous up-regulation of all Hsps. Up-regulation of the Hsps begins at the onset of diapause, persists throughout the overwintering period, and ceases within hours after the fly receives the signal to reinitiate development. The up-regulation of Hsps appears to be common to diapause in species representing diverse insect orders including Diptera, Lepidoptera, Coleoptera, and Hymenoptera as well as in diapauses that occur in different developmental stages (embryo, larva, pupa, adult). Suppressing expression of Hsp23 and Hsp70 in flies by using RNAi did not alter the decision to enter diapause or the duration of diapause, but it had a profound effect on the pupa's ability to survive low temperatures. We thus propose that up-regulation of Hsps during diapause is a major factor contributing to cold-hardiness of overwintering insects.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Tauber MJ, Tauber CA, Masaki S. Seasonal Adaptations of Insects. Oxford: Oxford Univ Press; 1986.
-
- Danks HV. Insect Dormancy: An Ecological Perspective. Ottawa, Canada: Biological Survey; 1987.
-
- Denlinger DL, Yocum GD, Rinehart JP. In: Comprehensive Insect Molecular Science. Gilbert LI, Iatrou K, Gill S, editors. Vol 3. Amsterdam: Elsevier; 2005. pp. 615–640.
-
- Denlinger DL. Annu Rev Entomol. 2002;47:93–122. - PubMed
-
- Lee RE. In: Insects at Low Temperature. Lee RE, Denlinger DL, editors. New York: Chapman & Hall; 1991. pp. 17–46.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

