Linking synaptic plasticity and spike output at excitatory and inhibitory synapses onto cerebellar Purkinje cells
- PMID: 17522301
- PMCID: PMC6672768
- DOI: 10.1523/JNEUROSCI.5117-06.2007
Linking synaptic plasticity and spike output at excitatory and inhibitory synapses onto cerebellar Purkinje cells
Abstract
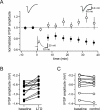
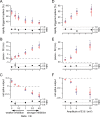
Understanding the relationship between synaptic plasticity and neuronal output is essential if we are to understand how plasticity is encoded in neural circuits. In the cerebellar cortex, motor learning is thought to be implemented by long-term depression (LTD) of excitatory parallel fiber (PF) to Purkinje cell synapses triggered by climbing fiber (CF) input. However, theories of motor learning generally neglect the contribution of plasticity of inhibitory inputs to Purkinje cells. Here we describe how CF-induced plasticity of both excitatory and inhibitory inputs is reflected in Purkinje cell spike output. We show that coactivation of the CF with PF input and interneuron input leads not only to LTD of PF synapses but also to comparable, "balanced" LTD of evoked inhibitory inputs. These two forms of plasticity have opposite effects on the spike output of Purkinje cells, with the number and timing of spikes sensitively reflecting the degree of plasticity. We used dynamic clamp to evaluate plasticity-induced changes in spike responses to sequences of excitation and feedforward inhibition of varied relative and absolute amplitude. Balanced LTD of both excitatory and inhibitory components decreased the net spike output of Purkinje cells only for inputs with small inhibitory components, whereas for inputs with a larger proportion of feedforward inhibition CF-triggered LTD resulted in an increase in the net spike output. Thus, the net effect of CF-triggered plasticity on Purkinje cell output depends on the balance of excitation and feedforward inhibition and can paradoxically increase cerebellar output, contrary to current theories of cerebellar motor learning.
Figures





References
-
- Albus J. A theory of cerebellar function. Math Biosci. 1971;28:167–171.
-
- Bell AJ, Mainen ZF, Sejnowski TJ. “Balancing” of conductances may explain irregularity of cortical spiking. La Jolla, CA: Institute for Neural Computation; 1995. p. 9502.
-
- Bell CC, Grimm RJ. Discharge properties of Purkinje cells recorded on single and double microelectrodes. J Neurophysiol. 1969;32:1044–1055. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
