Multiple modes of synaptic excitation of olfactory bulb granule cells
- PMID: 17522307
- PMCID: PMC6672747
- DOI: 10.1523/JNEUROSCI.4630-06.2007
Multiple modes of synaptic excitation of olfactory bulb granule cells
Abstract
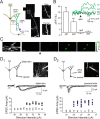
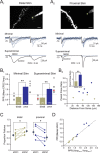
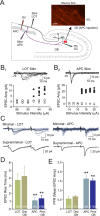
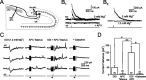
Inhibition generated by granule cells, the most common GABAergic cell type in the olfactory bulb, plays a critical role in shaping the output of the olfactory bulb. However, relatively little is known about the synaptic mechanisms responsible for activating these interneurons in addition to the specialized dendrodendritic synapses located on distal dendrites. Using two-photon guided minimal stimulation in acute rat brain slices, we found that distal and proximal excitatory synapses onto granule cells are functionally distinct. Proximal synapses arise from piriform cortical neurons and facilitate with paired-pulse stimulation, whereas distal dendrodendritic synapses generate EPSCs with slower kinetics that depress with paired stimulation. Proximal cortical feedback inputs can relieve the tonic Mg block of NMDA receptors (NMDARs) at distal synapses and gate dendrodendritic inhibition onto mitral cells. Most excitatory synapses we examined onto granule cells activated both NMDARs and AMPA receptors, whereas a subpopulation appeared to be NMDAR silent. The convergence of two types of excitatory inputs onto GABAergic granule cells provides a novel mechanism for regulating the degree of interglomerular processing of sensory input in the olfactory bulb through piriform cortex/olfactory bulb synaptic interactions.
Figures



References
-
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. - PubMed
-
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. - PubMed
-
- Chen WR, Xiong W, Shepherd GM. Analysis of relations between NMDA receptors and GABA release at olfactory bulb reciprocal synapses. Neuron. 2000;25:625–633. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
