Role of endogenous IFN-gamma in macrophage programming induced by IL-12 and IL-18
- PMID: 17523872
- PMCID: PMC2956645
- DOI: 10.1089/jir.2007.0128
Role of endogenous IFN-gamma in macrophage programming induced by IL-12 and IL-18
Abstract
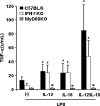
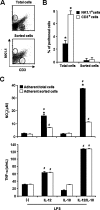
Besides the established role of interleukin-12 (IL-12) and IL-18 on interferon-gamma (IFN-gamma) production by natural killer (NK), T, and B cells, the effects of these cytokines on macrophages are largely unknown. Here, we investigated the role of IL-12/IL-18 on nitric oxide (NO) and tumor necrosis factor-alpha (TNF-alpha) production by CD11b(+) adherent peritoneal cells, focusing on the involvement of endogenously produced IFN-gamma. C57BL/6 cells released substantial amounts of NO when stimulated with IFN-gamma or lipopolysaccharide (LPS), but failed to respond to IL-12 or IL-18 or both. However, IL-12/IL-18 pretreatment was able to program these cells to release 6-8-fold more NO and TNF-alpha in response to LPS or Trypanosoma cruzi stimulation, with NO levels directly correlating with macrophage resistance to intracellular parasite growth. Analysis of IL-12/IL-18-primed cells from mice deficient in IFN-gamma, IFNGR, and IFN regulatory factor-1 (IRF-1) revealed that these molecules were essential for LPS-induced NO release, but TNF-alpha production was IFN-gamma independent. Conversely, the myeloid differentiation factor 88 (MyD88)-dependent pathway was indispensable for IL-12/IL-18-programmed LPS-induced TNF-alpha production, but not for NO release. Contaminant T and NK cells largely modulated the IL-12/IL-18 programming of LPS-induced NO response through IFN-gamma secretion. Nevertheless, a small population of IFN-gamma(+) cells with a macrophage phenotype was also identified, particularly in the peritoneum of chronically T. cruzi-infected mice, reinforcing the notion that macrophages can be an alternative source of IFN-gamma. Taken together, our data contribute to elucidate the molecular basis of the IL-12/IL-18 autocrine pathway of macrophage activation, showing that endogenous IFN-gamma plays an important role in programming the NO response, whereas the TNF-alpha response occurs through an IFN-gamma-independent pathway.
Figures

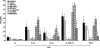
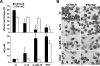

References
-
- Heufler C. Koch F. Stanzl U. Topar G. Wysocka M. Trinchieri G. Enk A. Steinman RM. Romani N. Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-γ production by T helper 1 cells. Eur. J. Immunol. 1996;26:659–668. - PubMed
-
- Stobie L. Gurunathan S. Prussin C. Sacks DL. Glaichenhaus N. Wu CY. Seder RA. The role of antigen and IL-12 in sustaining Th1 memory cells in vivo: IL-12 is required to maintain memory/effector Th1 cells sufficient to mediate protection to an infectious parasite challenge. Proc. Natl. Acad. Sci. USA. 2000;97:8427–8432. - PMC - PubMed
-
- Germann T. Bongartz M. Dlugonska H. Hess H. Schmitt E. Kolbe L. Kolsch E. Podlaski FJ. Gately MK. Rude E. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur. J. Immunol. 1995;25:823–829. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

