A review of the methodological features of systematic reviews in maternal medicine
- PMID: 17524137
- PMCID: PMC1910604
- DOI: 10.1186/1741-7015-5-10
A review of the methodological features of systematic reviews in maternal medicine
Abstract
Background: In maternal medicine, research evidence is scattered making it difficult to access information for clinical decision making. Systematic reviews of good methodological quality are essential to provide valid inferences and to produce usable evidence summaries to guide management. This review assesses the methodological features of existing systematic reviews in maternal medicine, comparing Cochrane and non-Cochrane reviews in maternal medicine.
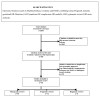
Methods: Medline, Embase, Database of Reviews of Effectiveness (DARE) and Cochrane Database of Systematic Reviews (CDSR) were searched for relevant reviews published between 2001 and 2006. We selected those reviews in which a minimum of two databases were searched and the primary outcome was related to the maternal condition. The selected reviews were assessed for information on framing of question, literature search and methods of review.
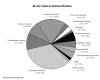
Results: Out of 2846 citations, 68 reviews were selected. Among these, 39 (57%) were Cochrane reviews. Most of the reviews (50/68, 74%) evaluated therapeutic interventions. Overall, 54/68 (79%) addressed a focussed question. Although 64/68 (94%) reviews had a detailed search description, only 17/68 (25%) searched without language restriction. 32/68 (47%) attempted to include unpublished data and 11/68 (16%) assessed for the risk of missing studies quantitatively. The reviews had deficiencies in the assessment of validity of studies and exploration for heterogeneity. When compared to Cochrane reviews, other reviews were significantly inferior in specifying questions (OR 20.3, 95% CI 1.1-381.3, p = 0.04), framing focussed questions (OR 30.9, 95% CI 3.7- 256.2, p = 0.001), use of unpublished data (OR 5.6, 95% CI 1.9-16.4, p = 0.002), assessment for heterogeneity (OR 38.1, 95%CI 2.1, 688.2, p = 0.01) and use of meta-analyses (OR 3.7, 95% CI 1.3-10.8, p = 0.02).
Conclusion: This study identifies areas which have a strong influence on maternal morbidity and mortality but lack good quality systematic reviews. Overall quality of the existing systematic reviews was variable. Cochrane reviews were of better quality as compared to other reviews. There is a need for good quality systematic reviews to inform practice in maternal medicine.
Figures
References
-
- Department of Health: Specialised Services National Definition Set: 4 Specialised services for women's health. . Department of health. 2006. http://www.dh.gov.uk/en/PolicyAndGuidance/HealthAndSocialCareTopics/Spec...
-
- Sacks HS, Reitman D, Pagano D. Meta-analysis: an update. Mt Sinai J Med. 1996;63:216–224. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical