Mechanisms of neurosteroid interactions with GABA(A) receptors
- PMID: 17524487
- PMCID: PMC2047817
- DOI: 10.1016/j.pharmthera.2007.03.004
Mechanisms of neurosteroid interactions with GABA(A) receptors
Abstract
Neuroactive steroids have some of their most potent actions by augmenting the function of GABA(A) receptors. Endogenous steroid actions on GABA(A) receptors may underlie important effects on mood and behavior. Exogenous neuroactive steroids have potential as anesthetics, anticonvulsants, and neuroprotectants. We have taken multiple approaches to understand more completely the interaction of neuroactive steroids with GABA(A) receptors. We have developed many novel steroid analogues in this effort. Recent work has resulted in synthesis of new enantiomer analogue pairs, novel ligands that probe various properties of the steroid pharmacophore, fluorescent neuroactive steroid analogues, and photoaffinity labels. Using these tools, combined with receptor binding and electrophysiological assays, we have begun to untangle the complexity of steroid actions at this important class of ligand-gated ion channel.
Figures



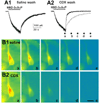


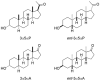
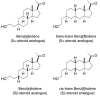

References
-
- Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB. α4β3δ GABAA receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem. 2001;276:38934–38939. - PubMed
-
- Agarwal K, Bali A, Gupta CM. Effect of phospholipid structure on stability and survival times of liposomes in circulation. Biochim Biophys Acta. 1986a;883:468–475. - PubMed
-
- Agarwal K, Bali A, Gupta CM. Influence of the phospholipid structure on the stability of liposomes in serum. Biochim Biophys Acta. 1986b;856:36–40. - PubMed
-
- Akabas MH. GABAA receptor structure-function studies: a reexamination in light of new acetylcholine receptor structures. Int Rev Neurobiol. 2004;62:1–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 NS057105/NS/NINDS NIH HHS/United States
- P01 GM047969/GM/NIGMS NIH HHS/United States
- R01 AA012951/AA/NIAAA NIH HHS/United States
- R01 MH078823/MH/NIMH NIH HHS/United States
- R01 AA012952/AA/NIAAA NIH HHS/United States
- NS54174/NS/NINDS NIH HHS/United States
- R21 AA014707/AA/NIAAA NIH HHS/United States
- GM47969/GM/NIGMS NIH HHS/United States
- MH78823/MH/NIMH NIH HHS/United States
- AA14707/AA/NIAAA NIH HHS/United States
- R01 NS054174/NS/NINDS NIH HHS/United States
- AA12951/AA/NIAAA NIH HHS/United States
- AA12952/AA/NIAAA NIH HHS/United States
- P01 AG010485/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources

