Membrane cholesterol modulates Kv1.5 potassium channel distribution and function in rat cardiomyocytes
- PMID: 17525113
- PMCID: PMC2075263
- DOI: 10.1113/jphysiol.2007.134809
Membrane cholesterol modulates Kv1.5 potassium channel distribution and function in rat cardiomyocytes
Abstract
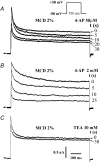
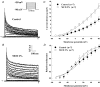
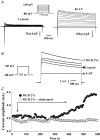
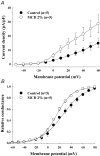
Membrane lipid composition is a major determinant of cell excitability. In this study, we assessed the role of membrane cholesterol composition in the distribution and function of Kv1.5-based channels in rat cardiac membranes. In isolated rat atrial myocytes, the application of methyl-beta-cyclodextrin (MCD), an agent that depletes membrane cholesterol, caused a delayed increase in the Kv1.5-based sustained component, I(kur), which reached steady state in approximately 7 min. This effect was prevented by preloading the MCD with cholesterol. MCD-increased current was inhibited by low 4-aminopyridine concentration. Neonatal rat cardiomyocytes transfected with Green Fluorescent Protein (GFP)-tagged Kv1.5 channels showed a large ultrarapid delayed-rectifier current (I(Kur)), which was also stimulated by MCD. In atrial cryosections, Kv1.5 channels were mainly located at the intercalated disc, whereas caveolin-3 predominated at the cell periphery. A small portion of Kv1.5 floated in the low-density fractions of step sucrose-gradient preparations. In live neonatal cardiomyocytes, GFP-tagged Kv1.5 channels were predominantly organized in clusters at the basal plasma membrane. MCD caused reorganization of Kv1.5 subunits into larger clusters that redistributed throughout the plasma membrane. The MCD effect on clusters was sizable 7 min after its application. We conclude that Kv1.5 subunits are concentrated in cholesterol-enriched membrane microdomains distinct from caveolae, and that redistribution of Kv1.5 subunits by depletion of membrane cholesterol increases their current-carrying capacity.
Figures



References
-
- Barbuti A, Gravante B, Riolfo M, Milanesi R, Terragni B, DiFrancesco D. Localization of pacemaker channels in lipid rafts regulates channel kinetics. Circ Res. 2004;94:1325–1331. - PubMed
-
- Boixel C, Gonzalez W, Louedec L, Hatem SN. Mechanisms of L-type Ca2+ current downregulation in rat atrial myocytes during heart failure. Circ Res. 2001;89:607–613. - PubMed
-
- Brainard AM, Miller AJ, Martens JR, England SK. Maxi-K channels localize to caveolae in human myometrium: a role for an actin-channel-caveolin complex in the regulation of myometrial smooth muscle K+ current. Am J Physiol Cell Physiol. 2005;289:C49–C57. - PubMed
-
- Christian AE, Haynes MP, Phillips MC, Rothblat GH. Use of cyclodextrins for manipulating cellular cholesterol content. J Lipid Res. 1997;38:2264–2272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

