The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus
- PMID: 17525114
- PMCID: PMC2075237
- DOI: 10.1113/jphysiol.2007.134460
The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus
Abstract
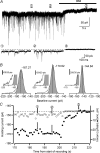
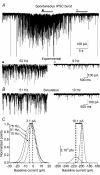
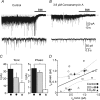
The extracellular space of the brain contains gamma-aminobutyric acid (GABA) that activates extrasynaptic GABA(A) receptors mediating tonic inhibition. The source of this GABA is uncertain: it could be overspill of vesicular release, non-vesicular leakage, reverse transport, dying cells or glia. Using a novel approach, we simultaneously measured phasic and tonic inhibitory currents and assessed their correlation. Enhancing or diminishing vesicular GABA release in hippocampal neurons caused highly correlated changes in the two inhibitions. During high-frequency phasic inhibitory bursts, tonic current was also enhanced as shown by simulating the summation of IPSCs and by recordings in knockout mice devoid of tonic inhibitory current. When vesicular release was reduced by blocking action potentials or the vesicular GABA transporter, phasic and tonic currents decreased in a correlated fashion. Our results are consistent with most of hippocampal tonic inhibitory current being mediated by GABA released from the very vesicles responsible for activating phasic inhibition.
Figures






References
-
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. - PubMed
-
- Brunig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002;443:43–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

