Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage
- PMID: 17525142
- PMCID: PMC2291147
- DOI: 10.1093/brain/awm095
Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage
Abstract
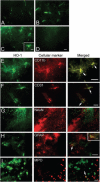
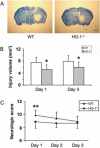
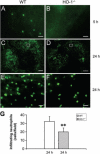
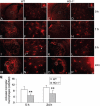
Because heme oxygenase (HO) is the rate limiting enzyme in the degradation of the pro-oxidant hemin/heme from blood, here we investigated the contribution of the inducible HO-1 to early brain injury produced by intracerebral haemorrhage (ICH). We found that after induction of ICH, HO-1 proteins were highly detectable in the peri-ICH region predominantly in microglia/macrophages and endothelial cells. Remarkably, the injury volume was significantly smaller in HO-1 knockout (HO-1-/-) mice than in wild-type controls 24 and 72 h after ICH. Although the brain water content did not appear to be significantly different, the protection in HO-1-/- mice was associated with a marked reduction in ICH-induced leucocyte infiltration, microglia/macrophage activation and free radical levels. These data reveal a previously unrecognized role of HO-1 in early brain injury after ICH. Thus, modulation of HO-1 signalling should be assessed further in clinical settings, especially for haemorrhagic states.
Figures


References
-
- Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res. 2005;27:268–79. - PubMed
-
- Chen K, Gunter K, Maines MD. Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J Neurochem. 2000;75:304–13. - PubMed
-
- Clark W, Gunion-Rinker L, Lessov N, Hazel K. Citicoline treatment for experimental intracerebral hemorrhage in mice. Stroke. 1998;29:2136–40. - PubMed

