The myofibroblast: one function, multiple origins
- PMID: 17525249
- PMCID: PMC1899462
- DOI: 10.2353/ajpath.2007.070112
The myofibroblast: one function, multiple origins
Abstract
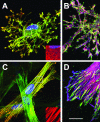
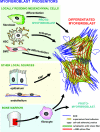
The crucial role played by the myofibroblast in wound healing and pathological organ remodeling is well established; the general mechanisms of extracellular matrix synthesis and of tension production by this cell have been amply clarified. This review discusses the pattern of myofibroblast accumulation and fibrosis evolution during lung and liver fibrosis as well as during atheromatous plaque formation. Special attention is paid to the specific features characterizing each of these processes, including the spectrum of different myofibroblast precursors and the distinct pathways involved in the formation of differentiated myofibroblasts in each lesion. Thus, whereas in lung fibrosis it seems that most myofibroblasts derive from resident fibroblasts, hepatic stellate cells are the main contributor for liver fibrosis and media smooth muscle cells are the main contributor for the atheromatous plaque. A better knowledge of the molecular mechanisms conducive to the appearance of differentiated myofibroblasts in each pathological situation will be useful for the understanding of fibrosis development in different organs and for the planning of strategies aiming at their prevention and therapy.
Figures

References
-
- Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. - PubMed
-
- Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. - PubMed
-
- Desmoulière A, Darby IA, Gabbiani G. Normal and pathologic soft tissue remodeling: role of the myofibroblast, with special emphasis on liver and kidney fibrosis. Lab Invest. 2003;83:1689–1707. - PubMed
-
- De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–447. - PubMed
-
- Thannickal VJ, Toews GB, White ES, Lynch JP, 3rd, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. - PubMed
Publication types
MeSH terms
Grants and funding
- HL 67967/HL/NHLBI NIH HHS/United States
- P01 HL031963/HL/NHLBI NIH HHS/United States
- R37 HL028737/HL/NHLBI NIH HHS/United States
- HL 31963/HL/NHLBI NIH HHS/United States
- HL 77297 S/HL/NHLBI NIH HHS/United States
- R01 HL067967/HL/NHLBI NIH HHS/United States
- HL 52285/HL/NHLBI NIH HHS/United States
- P50 HL074024/HL/NHLBI NIH HHS/United States
- R01 HL052285/HL/NHLBI NIH HHS/United States
- R01 HL077297/HL/NHLBI NIH HHS/United States
- HL 28737/HL/NHLBI NIH HHS/United States
- R01 HL028737/HL/NHLBI NIH HHS/United States
- HL 74024/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

