Genetic mapping in mice identifies DMBT1 as a candidate modifier of mammary tumors and breast cancer risk
- PMID: 17525270
- PMCID: PMC1899446
- DOI: 10.2353/ajpath.2007.060512
Genetic mapping in mice identifies DMBT1 as a candidate modifier of mammary tumors and breast cancer risk
Abstract
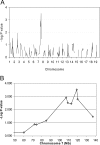
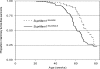
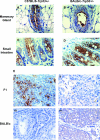
Low-penetrance breast cancer susceptibility alleles seem to play a significant role in breast cancer risk but are difficult to identify in human cohorts. A genetic screen of 176 N2 backcross progeny of two Trp53(+/-) strains, BALB/c and C57BL/6, which differ in their susceptibility to mammary tumors, identified a modifier of mammary tumor susceptibility in an approximately 25-Mb interval on mouse chromosome 7 (designated SuprMam1). Relative to heterozygotes, homozygosity for BALB/c alleles of SuprMam1 significantly decreased mammary tumor latency from 70.7 to 61.1 weeks and increased risk twofold (P = 0.002). Dmbt1 (deleted in malignant brain tumors 1) was identified as a candidate modifier gene within the SuprMam1 interval because it was differentially expressed in mammary tissues from BALB/c-Trp53(+/-) and C57BL/6-Trp53(+/-) mice. Dmbt1 mRNA and protein was reduced in mammary glands of the susceptible BALB/c mice. Immunohistochemical staining demonstrated that DMBT1 protein expression was also significantly reduced in normal breast tissue from women with breast cancer (staining score, 1.8; n = 46) compared with cancer-free controls (staining score, 3.9; n = 53; P < 0.0001). These experiments demonstrate the use of Trp53(+/-) mice as a sensitized background to screen for low-penetrance modifiers of cancer. The results identify a novel mammary tumor susceptibility locus in mice and support a role for DMBT1 in suppression of mammary tumors in both mice and women.
Figures
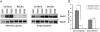

References
-
- Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. - PubMed
-
- Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, Bishop DT, Weber B, Lenoir G, Chang-Claude J, Sobol H, Teare MD, Struewing J, Arason A, Scherneck S, Peto J, Rebbeck TR, Tonin P, Neu-hausen S, Barkardottir R, Eyfjord J, Lynch H, Ponder BA, Gayther SA, Zelada-Hedman M, The Breast Cancer Linkage Consortiu Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. Am J Hum Genet. 1998;62:676–689. - PMC - PubMed
-
- Newman B, Mu H, Butler LM, Millikan RC, Moorman PG, King MC. Frequency of breast cancer attributable to BRCA1 in a population-based series of American women. JAMA. 1998;279:915–921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

