p140Cap protein suppresses tumour cell properties, regulating Csk and Src kinase activity
- PMID: 17525734
- PMCID: PMC1894765
- DOI: 10.1038/sj.emboj.7601724
p140Cap protein suppresses tumour cell properties, regulating Csk and Src kinase activity
Abstract
We recently identified p140Cap as a novel adaptor protein, expressed in epithelial-rich tissues and phosphorylated upon cell matrix adhesion and growth factor treatment. Here, we characterise p140Cap as a novel Src-binding protein, which regulates Src activation via C-terminal Src kinase (Csk). p140Cap silencing increases cell spreading, migration rate and Src kinase activity. Accordingly, increased expression of p140Cap activates Csk, leading to inhibition of Src and downstream signalling as well as of cell motility and invasion. Moreover, cell proliferation and "in vivo" breast cancer cell growth are strongly impaired by high levels of p140Cap, providing the first evidence that p140Cap is a novel negative regulator of tumour growth.
Figures
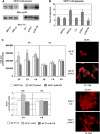



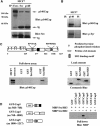




References
-
- Avizienyte E, Wyke AW, Jones RJ, McLean GW, Westhoff MA, Brunton VG, Frame MC (2002) Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat Cell Biol 4: 632–638 - PubMed
-
- Bon G, Folgiero V, Bossi G, Felicioni L, Marchetti A, Sacchi A, Falcioni R (2006) Loss of beta4 integrin subunit reduces the tumorigenicity of MCF7 mammary cells and causes apoptosis upon hormone deprivation. Clin Cancer Res 12: 3280–3287 - PubMed
-
- Bouton AH, Riggins RB, Bruce-Staskal PJ (2001) Functions of the adapter protein Cas: signal convergence and the determination of cellular responses. Oncogene 20: 6448–6458 - PubMed
-
- Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

