Pancreas-specific RelA/p65 truncation increases susceptibility of acini to inflammation-associated cell death following cerulein pancreatitis
- PMID: 17525802
- PMCID: PMC1868784
- DOI: 10.1172/JCI29882
Pancreas-specific RelA/p65 truncation increases susceptibility of acini to inflammation-associated cell death following cerulein pancreatitis
Abstract
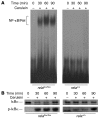
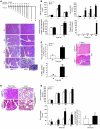
Activation of the transcription factor NF-kappaB/Rel has been shown to be involved in inflammatory disease. Here we studied the role of RelA/p65, the main transactivating subunit, during acute pancreatitis using a Cre-loxP strategy. Selective truncation of the rela gene in pancreatic exocrine cells led to both severe injury of the acinar cells and systemic complications including lung and liver damage. Our data demonstrated that expression and induction of the protective pancreas-specific acute phase protein pancreatitis-associated protein 1 (PAP1) depended on RelA/p65. Lentiviral gene transfer of PAP1 cDNA reduced the extent of necrosis and infiltration in the pancreata of mice with selective truncation of RelA/p65. These results provide in vivo evidence for RelA/p65 protection of acinar cell death via upregulation of PAP1. Moreover, our data underscore the pancreas-specific role of NF-kappaB/Rel and suggest multidimensional roles of NF-kappaB/Rel in different cells and contexts during inflammation.
Figures
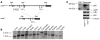







Comment in
- J Clin Invest. 117:1502.
References
-
- Baron T.H., Morgan D.E. Acute necrotizing pancreatitis. N. Engl. J. Med. 1999;340:1412–1417. - PubMed
-
- Whitcomb D.C. Clinical practice. Acute pancreatitis. N. Engl. J. Med. 2006;354:2142–2150. - PubMed
-
- Geokas M.C., Rinderknecht H. Free proteolytic enzymes in pancreatic juice of patients with acute pancreatitis. Am. J. Dig. Dis. 1974;19:591–598. - PubMed
-
- Bialek R., Willemer S., Arnold R., Adler G. Evidence of intracellular activation of serine proteases in acute cerulein-induced pancreatitis in rats. Scand. J. Gastroenterol. 1991;26:190–196. - PubMed
-
- Naruse S. Molecular pathophysiology of pancreatitis. Intern. Med. 2003;42:288–289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

