Phosphorylation in the serine/threonine 2609-2647 cluster promotes but is not essential for DNA-dependent protein kinase-mediated nonhomologous end joining in human whole-cell extracts
- PMID: 17526517
- PMCID: PMC1919499
- DOI: 10.1093/nar/gkm339
Phosphorylation in the serine/threonine 2609-2647 cluster promotes but is not essential for DNA-dependent protein kinase-mediated nonhomologous end joining in human whole-cell extracts
Abstract
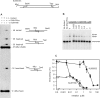
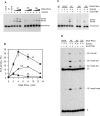
Previous work suggested that phosphorylation of DNA-PKcs at several serine/threonine (S/T) residues at positions 2609-2647 promotes DNA-PK-dependent end joining. In an attempt to clarify the role of such phosphorylation, end joining was examined in extracts of DNA-PKcs-deficient M059J cells. Joining of ends requiring gap filling prior to ligation was completely dependent on complementation of these extracts with exogenous DNA-PKcs. DNA-PKcs with either S/T --> A or S/T --> D substitutions at all six sites in the 2609-2647 cluster also supported end joining, but with markedly lower efficiency than wild-type protein. The residual end joining was greater with the S/T --> D-substituted than with the S/T --> A-substituted protein. A specific inhibitor of the kinase activity of DNA-PK, KU57788, completely blocked end joining promoted by wild type as well as both mutant forms of DNA-PK, while inhibition of ATM kinase did not. The fidelity of end joining was not affected by the mutant DNA-PKcs alleles or the inhibitors. Overall, the results support a role for autophosphorylation of the 2609-2647 cluster in promoting end joining and controlling the accessibility of DNA ends, but suggest that DNA-PK-mediated phosphorylation at other sites, on either DNA-PKcs or other proteins, is at least as important as the 2609-2647 cluster in regulating end joining.
Figures
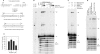

References
-
- Valerie K, Povirk LF. Regulation and mechanisms of mammalian double-strand break repair. Oncogene. 2003;22:5792–5812. - PubMed
-
- Meek K, Gupta S, Ramsden DA, Lees-Miller SP. The DNA-dependent protein kinase: the director at the end. Immunol. Rev. 2004;200:132–141. - PubMed
-
- Douglas P, Gupta S, Morrice N, Meek K, Lees-Miller SP. DNA-PK-dependent phosphorylation of Ku70/80 is not required for non-homologous end joining. DNA Repair. 2005;4:1006–1018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

