Cell cycle regulation as a mechanism for functional separation of the apparently redundant uracil DNA glycosylases TDG and UNG2
- PMID: 17526518
- PMCID: PMC1920262
- DOI: 10.1093/nar/gkm337
Cell cycle regulation as a mechanism for functional separation of the apparently redundant uracil DNA glycosylases TDG and UNG2
Abstract
Human Thymine-DNA Glycosylase (TDG) is a member of the uracil DNA glycosylase (UDG) superfamily. It excises uracil, thymine and a number of chemical base lesions when mispaired with guanine in double-stranded DNA. These activities are not unique to TDG; at least three additional proteins with similar enzymatic properties are present in mammalian cells. The successful co-evolution of these enzymes implies the existence of non-redundant biological functions that must be coordinated. Here, we report cell cycle regulation as a mechanism for the functional separation of apparently redundant DNA glycosylases. We show that cells entering S-phase eliminate TDG through the ubiquitin-proteasome system and then maintain a TDG-free condition until G2. Incomplete degradation of ectopically expressed TDG impedes S-phase progression and cell proliferation. The mode of cell cycle regulation of TDG is strictly inverse to that of UNG2, which peaks in and throughout S-phase and then declines to undetectable levels until it appears again just before the next S-phase. Thus, TDG- and UNG2-dependent base excision repair alternates throughout the cell cycle, and the ubiquitin-proteasome pathway constitutes the underlying regulatory system.
Figures


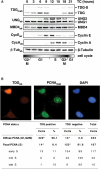


References
-
- Krokan HE, Drablos F, Slupphaug G. Uracil in DNA – occurrence, consequences and repair. Oncogene. 2002;21:8935–8948. - PubMed
-
- Barnes DE, Lindahl T. Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu. Rev. Genet. 2004;38:445–476. - PubMed
-
- Slupphaug G, Eftedal I, Kavli B, Bharati S, Helle NM, Haug T, Levine DW, Krokan HE. Properties of a recombinant human uracil-DNA glycosylase from the UNG gene and evidence that UNG encodes the major uracil-DNA glycosylase. Biochemistry. 1995;34:128–138. - PubMed
-
- Haushalter KA, Todd Stukenberg MW, Kirschner MW, Verdine GL. Identification of a new uracil-DNA glycosylase family by expression cloning using synthetic inhibitors. Curr. Biol. 1999;9:174–185. - PubMed
-
- Hendrich B, Hardeland U, Ng HH, Jiricny J, Bird A. The thymine glycosylase MBD4 can bind to the product of deamination at methylated CpG sites. Nature. 1999;401:301–304. - PubMed

