Flexibility in HIV-1 assembly subunits: solution structure of the monomeric C-terminal domain of the capsid protein
- PMID: 17526561
- PMCID: PMC1929042
- DOI: 10.1529/biophysj.106.101089
Flexibility in HIV-1 assembly subunits: solution structure of the monomeric C-terminal domain of the capsid protein
Abstract
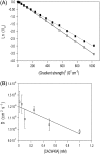
The protein CA forms the mature capsid of human immunodeficiency virus. Hexamerization of the N-terminal domain and dimerization of the C-terminal domain, CAC, occur during capsid assembly, and both domains constitute potential targets for anti-HIV inhibitors. CAC homodimerization occurs mainly through its second helix, and is abolished when its sole tryptophan is mutated to alanine. Previous thermodynamic data obtained with the dimeric and monomeric forms of CAC indicate that the structure of the mutant resembles that of a monomeric intermediate found in the folding and association reactions of CAC. We have solved the three-dimensional structure in aqueous solution of the monomeric mutant. The structure is similar to that of the subunits in the dimeric, nonmutated CAC, except the segment corresponding to the second helix, which is highly dynamic. At the end of this region, the polypeptide chain is bent to bury several hydrophobic residues and, as a consequence, the last two helices are rotated 90 degrees when compared to their position in dimeric CAC. The previously obtained thermodynamic data are consistent with the determined structure of the monomeric mutant. This extraordinary ability of CAC to change its structure may contribute to the different modes of association of CA during HIV assembly, and should be taken into account in the design of new drugs against this virus.
Figures

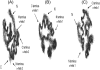

References
-
- Freed, E. O. 1998. HIV-1 Gag proteins: diverse functions in the virus life cycle. Virology. 251:1–15. - PubMed
-
- Freed, E. O., and M. Martin. 2001. HIVs and their replication. In Field Virology. D. M. Knipe and P. M. Howley, editors. Lippincott, Philadelphia, PA.
-
- Vogt, V. M. 1996. Proteolytic processing and particle maturation. Curr. Top. Microbiol. Immunol. 214:95–132. - PubMed
-
- Fuller, T. W., T. Wilk, B. E. Gowen, H.-G. Kräusslich, and V. M. Vogt. 1997. Cryo-electron microscopy reveals ordered domains in the immature HIV-1 particle. Curr. Biol. 7:729–738. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

