Determination of the contribution of the myristoyl group and hydrophobic amino acids of recoverin on its dynamics of binding to lipid monolayers
- PMID: 17526567
- PMCID: PMC1959526
- DOI: 10.1529/biophysj.106.103481
Determination of the contribution of the myristoyl group and hydrophobic amino acids of recoverin on its dynamics of binding to lipid monolayers
Abstract
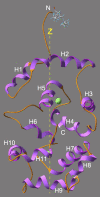
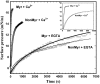
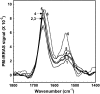
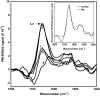
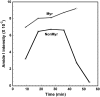
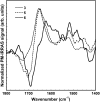
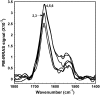
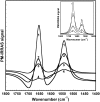
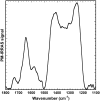
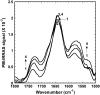
It has been postulated that myristoylation of peripheral proteins would facilitate their binding to membranes. However, the exact involvement of this lipid modification in membrane binding is still a matter of debate. Proteins containing a Ca(2+)-myristoyl switch where the extrusion of their myristoyl group is dependent on calcium binding is best illustrated by the Ca(2+)-binding recoverin, which is present in retinal rod cells. The parameters responsible for the modulation of the membrane binding of recoverin are still largely unknown. This study was thus performed to determine the involvement of different parameters on recoverin membrane binding. We have used surface pressure measurements and PM-IRRAS spectroscopy to monitor the adsorption of myristoylated and nonmyristoylated recoverin onto phospholipid monolayers in the presence and absence of calcium. The adsorption curves have shown that the myristoyl group and hydrophobic residues of myristoylated recoverin strongly accelerate membrane binding in the presence of calcium. In the case of nonmyristoylated recoverin in the presence of calcium, hydrophobic residues alone are responsible for its much faster monolayer binding than myristoylated and nonmyristoylated recoverin in the absence of calcium. The infrared spectra revealed that myristoylated and nonmyristoylated recoverin behave very different upon adsorption onto phospholipid monolayers. Indeed, PM-IRRAS spectra indicated that the myristoyl group allows a proper orientation and organization as well as faster and stronger binding of myristoylated recoverin to lipid monolayers compared to nonmyristoylated recoverin. Simulations of the spectra have allowed us to postulate that nonmyristoylated recoverin changes conformation and becomes hydrated at large extents of adsorption as well as to estimate the orientation of myristoylated recoverin with respect to the monolayer plane. In addition, adsorption measurements and electrophoresis of trypsin-treated myristoylated recoverin in the presence of zinc or calcium demonstrated that recoverin has a different conformation but a similar extent of monolayer binding in the presence of such ions.
Figures




References
-
- Kinnunen, P. K. J., A. Kõiv, J. Y. A. Lehtonen, M. Rytömaa, and P. Mustonen. 1994. Lipid dynamics and peripheral interactions of proteins with membrane surfaces. Chem. Phys. Lipids. 73:181–207. - PubMed
-
- Marsh, D., and T. Páli. 2004. The protein-lipid interface: perspectives from magnetic resonance and crystal structures. Biochim. Biophys. Acta. 1666:118–141. - PubMed
-
- Grenier, S., P. Desmeules, A. K. Dutta, A. Yamazaki, and C. Salesse. 1998. Determination of the depth of penetration of the α subunit of retinal G protein in membranes: a spectroscopic study. Biochim. Biophys. Acta. 1370:199–206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

