Steric selectivity in Na channels arising from protein polarization and mobile side chains
- PMID: 17526571
- PMCID: PMC1959557
- DOI: 10.1529/biophysj.107.105478
Steric selectivity in Na channels arising from protein polarization and mobile side chains
Abstract
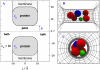
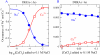
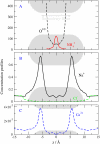
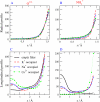
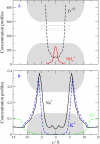
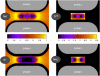
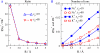
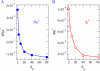
Monte Carlo simulations of equilibrium selectivity of Na channels with a DEKA locus are performed over a range of radius R and protein dielectric coefficient epsilon(p). Selectivity arises from the balance of electrostatic forces and steric repulsion by excluded volume of ions and side chains of the channel protein in the highly concentrated and charged (approximately 30 M) selectivity filter resembling an ionic liquid. Ions and structural side chains are described as mobile charged hard spheres that assume positions of minimal free energy. Water is a dielectric continuum. Size selectivity (ratio of Na+ occupancy to K+ occupancy) and charge selectivity (Na+ to Ca2+) are computed in concentrations as low as 10(-5) M Ca2+. In general, small R reduces ion occupancy and favors Na+ over K+ because of steric repulsion. Small epsilon(p) increases occupancy and favors Na+ over Ca2+ because protein polarization amplifies the pore's net charge. Size selectivity depends on R and is independent of epsilon(p); charge selectivity depends on both R and epsilon(p). Thus, small R and epsilon(p) make an efficient Na channel that excludes K+ and Ca2+ while maximizing Na+ occupancy. Selectivity properties depend on interactions that cannot be described by qualitative or verbal models or by quantitative models with a fixed free energy landscape.
Figures


References
-
- Hodgkin, A., A. Huxley, and B. Katz. 1949. Ionic currents underlying activity in the giant axon of the squid. Arch. Sci. Physiol. (Paris). 3:129–150.
-
- Dixon, M., and E. C. Webb. 1979. Enzymes. Academic Press, New York.
-
- Fersht, A. 1985. Enzyme Structure and Mechanism. Freeman, New York.
-
- Hille, B. 2001. Ionic Channels of Excitable Membranes. Sinauer Associates, Sunderland, MA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

