Competing docking interactions can bring about bistability in the MAPK cascade
- PMID: 17526574
- PMCID: PMC1965452
- DOI: 10.1529/biophysj.107.109132
Competing docking interactions can bring about bistability in the MAPK cascade
Abstract
Mitogen-activated protein kinases are crucial regulators of various cell fate decisions including proliferation, differentiation, and apoptosis. Depending on the cellular context, the Raf-Mek-Erk mitogen-activated protein kinase cascade responds to extracellular stimuli in an all-or-none manner, most likely due to bistable behavior. Here, we describe a previously unrecognized positive-feedback mechanism that emerges from experimentally observed sequestration effects in the core Raf-Mek-Erk cascade. Unphosphorylated/monophosphorylated Erk sequesters Mek into Raf-inaccessible complexes upon weak stimulation, and thereby inhibits cascade activation. Mek, once phosphorylated by Raf, triggers Erk phosphorylation, which in turn induces dissociation of Raf-inaccessible Mek-Erk heterodimers, and thus further amplifies Mek phosphorylation. We show that this positive circuit can bring about bistability for parameter values measured experimentally in living cells. Previous studies revealed that bistability can also arise from enzyme depletion effects in the Erk double (de)phosphorylation cycle. We demonstrate that the feedback mechanism proposed in this article synergizes with such enzyme depletion effects to bring about a much larger bistable range than either mechanism alone. Our results show that stable docking interactions and competition effects, which are common in protein kinase cascades, can result in sequestration-based feedback, and thus can have profound effects on the qualitative behavior of signaling pathways.
Figures


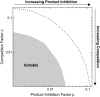

References
-
- Bhalla, U. S., P. T. Ram, and R. Iyengar. 2002. MAP kinase phosphatase as a locus of flexibility in a mitogen-activated protein kinase signaling network. Science. 297:1018–1023. - PubMed
-
- Bagowski, C. P., and J. E. Ferrell, Jr. 2001. Bistability in the JNK cascade. Curr. Biol. 11:1176–1182. - PubMed
-
- Ferrell, J. E., Jr., and E. M. Machleder. 1998. The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science. 280:895–898. - PubMed
-
- Paliwal, S., P. A. Iglesias, K. Campbell, Z. Hilioti, A. Groisman, and A. Levchenko. 2007. MAPK-mediated bimodal gene expression and adaptive gradient sensing in yeast. Nature. 446:46–51. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

