Effects of cholesterol on dry bilayers: interactions between phosphatidylcholine unsaturation and glycolipid or free sugar
- PMID: 17526577
- PMCID: PMC1929023
- DOI: 10.1529/biophysj.107.108886
Effects of cholesterol on dry bilayers: interactions between phosphatidylcholine unsaturation and glycolipid or free sugar
Abstract
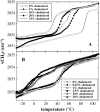
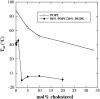
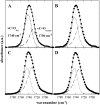
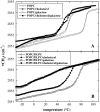
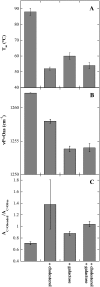
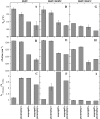
Cholesterol and other sterols are important components of biological membranes and are known to strongly influence the physical characteristics of lipid bilayers. Although this has been studied extensively in fully hydrated membranes, little is known about the effects of cholesterol on the stability of membranes in the dry state. Here, we present a Fourier transform infrared spectroscopy study on the effects of cholesterol on the phase behavior of dry liposomes composed of phosphatidylcholines with different degrees of fatty acid unsaturation or of mixtures of phosphatidylcholine with a plant galactolipid. In addition, we have analyzed the H-bonding of cholesterol, galactose, and a combination of the two additives to the P=O and C=O groups in dry phosphatidylcholine bilayers. The data indicate a complex balance of interactions between the different components in the dry state and a strong influence of fatty acid unsaturation on the interactions of the diacyl lipids with both cholesterol and galactose.
Figures




References
-
- Ohvo-Reikila, H., B. Ramstedt, P. Leppimaki, and J. P. Slotte. 2002. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 41:66–97. - PubMed
-
- Yeagle, P. L. 1985. Cholesterol and cell membranes. Biochim. Biophys. Acta. 822:267–287. - PubMed
-
- Barenholz, Y. 2002. Cholesterol and other membrane active sterols: from membrane evolution to “rafts”. Prog. Lipid Res. 41:1–5. - PubMed
-
- Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111–136. - PubMed
-
- Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature. 387:569–572. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

