Effect of cholesterol on the biophysical and physiological properties of a clinical pulmonary surfactant
- PMID: 17526587
- PMCID: PMC1929052
- DOI: 10.1529/biophysj.106.099762
Effect of cholesterol on the biophysical and physiological properties of a clinical pulmonary surfactant
Abstract
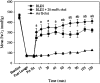
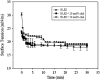
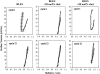
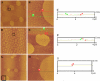
Pulmonary surfactant is a complex mixture of lipids and proteins that forms a surface-active film at the air-water interface of alveoli capable of reducing surface tension to near 0 mN/m. The role of cholesterol, the major neutral lipid component of pulmonary surfactant, remains uncertain. We studied the physiological effect of cholesterol by monitoring blood oxygenation levels of surfactant-deficient rats treated or not treated with bovine lipid extract surfactant (BLES) containing zero or physiological amounts of cholesterol. Our results indicate no significant difference between BLES and BLES containing cholesterol immediately after treatment; however, during ventilation, BLES-treated animals maintained higher PaO2 values compared to BLES+cholesterol-treated animals. We used a captive bubble tensiometer to show that physiological amounts of cholesterol do not have a detrimental effect on the surface activity of BLES at 37 degrees C. The effect of cholesterol on topography and lateral organization of BLES Langmuir-Blodgett films was also investigated using atomic force microscopy. Our data indicate that cholesterol induces the formation of domains within liquid-ordered domains (Lo). We used time-of-flight-secondary ion mass spectrometry and principal component analysis to show that cholesterol is concentrated in the Lo phase, where it induces structural changes.
Figures



References
-
- Lewis, J. F., and R. Veldhuizen. 2003. The role of exogenous surfactant in the treatment of acute lung injury. Annu. Rev. Physiol. 65:613–642. - PubMed
-
- Robertson, B., and H. L. Halliday. 1998. Principles of surfactant replacement. Biochim. Biophys. Acta. 1408:346–361. - PubMed
-
- Possmayer, F. 1997. Physicochemical aspects of pulmonary surfactant. In Fetal and Neonatal Physiology. R. A. Polin and W. W. Fox, editors. Saunders, Philadelphia. 1259–1275.
-
- Postle, A. D., E. L. Heeley, and D. C. Wilton. 2001. A comparison of the molecular species compositions of mammalian lung surfactant phospholipids. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 129:65–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

