CsrA inhibits translation initiation of Escherichia coli hfq by binding to a single site overlapping the Shine-Dalgarno sequence
- PMID: 17526692
- PMCID: PMC1951803
- DOI: 10.1128/JB.00529-07
CsrA inhibits translation initiation of Escherichia coli hfq by binding to a single site overlapping the Shine-Dalgarno sequence
Abstract
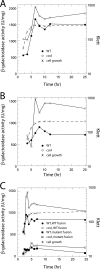
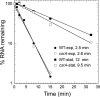
Csr (carbon storage regulation) of Escherichia coli is a global regulatory system that consists of CsrA, a homodimeric RNA binding protein, two noncoding small RNAs (sRNAs; CsrB and CsrC) that function as CsrA antagonists by sequestering this protein, and CsrD, a specificity factor that targets CsrB and CsrC for degradation by RNase E. CsrA inhibits translation initiation of glgC, cstA, and pgaA by binding to their leader transcripts and preventing ribosome binding. Translation inhibition is thought to contribute to the observed mRNA destabilization. Each of the previously known target transcripts contains multiple CsrA binding sites. A position-specific weight matrix search program was developed using known CsrA binding sites in mRNA. This search tool identified a potential CsrA binding site that overlaps the Shine-Dalgarno sequence of hfq, a gene that encodes an RNA chaperone that mediates sRNA-mRNA interactions. This putative CsrA binding site matched the SELEX-derived binding site consensus sequence in 8 out of 12 positions. Results from gel mobility shift and footprint assays demonstrated that CsrA binds specifically to this site in the hfq leader transcript. Toeprint and cell-free translation results indicated that bound CsrA inhibits Hfq synthesis by competitively blocking ribosome binding. Disruption of csrA caused elevated expression of an hfq'-'lacZ translational fusion, while overexpression of csrA inhibited expression of this fusion. We also found that hfq mRNA is stabilized upon entry into stationary-phase growth by a CsrA-independent mechanism. The interaction of CsrA with hfq mRNA is the first example of a CsrA-regulated gene that contains only one CsrA binding site.
Figures




References
-
- Babitzke, P., and T. Romeo. 2007. CsrB sRNA family: sequestration of RNA-binding regulatory proteins. Curr. Opin. Microbiol. 10:156-163. - PubMed
-
- Baker, C. S., I. Morozov, K. Suzuki, T. Romeo, and P. Babitzke. 2002. CsrA regulates glycogen biosynthesis by preventing translation of glgC in Escherichia coli. Mol. Microbiol. 44:1599-1610. - PubMed
-
- Bevilacqua, J. M., and P. C. Bevilacqua. 1998. Thermodynamic analysis of an RNA combinatorial library contained in a short hairpin. Biochemistry 37:15877-15884. - PubMed
-
- Bolivar, F., R. L. Rodriguez, P. J. Greene, M. C. Betlach, H. L. Heyneker, and H. W. Boyer. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95-113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

