Interactions between the lipoprotein PilP and the secretin PilQ in Neisseria meningitidis
- PMID: 17526700
- PMCID: PMC1951802
- DOI: 10.1128/JB.00060-07
Interactions between the lipoprotein PilP and the secretin PilQ in Neisseria meningitidis
Abstract
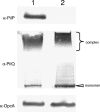
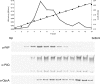
Neisseria meningitidis can be the causative agent of meningitis or septicemia. This bacterium expresses type IV pili, which mediate a variety of functions, including autoagglutination, twitching motility, biofilm formation, adherence, and DNA uptake during transformation. The secretin PilQ supports type IV pilus extrusion and retraction, but it also requires auxiliary proteins for its assembly and localization in the outer membrane. Here we have studied the physical properties of the lipoprotein PilP and examined its interaction with PilQ. We found that PilP was an inner membrane protein required for pilus expression and transformation, since pilP mutants were nonpiliated and noncompetent. These mutant phenotypes were restored by the expression of PilP in trans. The pilP gene is located upstream of pilQ, and analysis of their transcripts indicated that pilP and pilQ were cotranscribed. Furthermore, analysis of the level of PilQ expression in pilP mutants revealed greatly reduced amounts of PilQ only in the deletion mutant, exhibiting a polar effect on pilQ transcription. In vitro experiments using recombinant fragments of PilP and PilQ showed that the N-terminal region of PilP interacted with the middle part of the PilQ polypeptide. A three-dimensional reconstruction of the PilQ-PilP interacting complex was obtained at low resolution by transmission electron microscopy, and PilP was shown to localize around the cap region of the PilQ oligomer. These findings suggest a role for PilP in pilus biogenesis. Although PilQ does not need PilP for its stabilization or membrane localization, the specific interaction between these two proteins suggests that they might have another coordinated activity in pilus extrusion/retraction or related functions.
Figures





References
-
- Alm, R. A., and J. S. Mattick. 1997. Genes involved in the biogenesis and function of type-4 fimbriae in Pseudomonas aeruginosa. Gene 192:89-98. - PubMed
-
- Bayan, N., I. Guilvout, and A. P. Pugsley. 2006. Secretins take shape. Mol. Microbiol. 60:1-4. - PubMed
-
- Bitter, W. 2003. Secretins of Pseudomonas aeruginosa: large holes in the outer membrane. Arch. Microbiol. 179:307-314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

