Hypoxia-inducible factor linked to differential kidney cancer risk seen with type 2A and type 2B VHL mutations
- PMID: 17526729
- PMCID: PMC1952077
- DOI: 10.1128/MCB.00282-07
Hypoxia-inducible factor linked to differential kidney cancer risk seen with type 2A and type 2B VHL mutations
Abstract
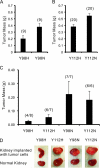
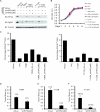
Clear cell carcinoma of the kidney is a major cause of mortality in patients with von Hippel-Lindau (VHL) disease, which is caused by germ line mutations that inactivate the VHL tumor suppressor gene. Biallelic VHL inactivation, due to mutations or hypermethylation, is also common in sporadic clear cell renal carcinomas. The VHL gene product, pVHL, is part of a ubiquitin ligase complex that targets the alpha subunits of the heterodimeric transcription factor hypoxia-inducible factor (HIF) for destruction under well-oxygenated conditions. All VHL mutations linked to classical VHL disease compromise this pVHL function although some missense mutations result in a low risk of kidney cancer (type 2A VHL disease) while others result in a high risk (type 2B VHL disease). We found that type 2A mutants were less defective than type 2B mutants when reintroduced into VHL-/- renal carcinoma cells with respect to HIF regulation. A stabilized version of HIF2alpha promoted tumor growth by VHL-/- cells engineered to produce type 2A mutants, while knock-down of HIF2alpha in cells producing type 2B mutants had the opposite effect. Therefore, quantitative differences with respect to HIF deregulation are sufficient to account for the differential risks of kidney cancer linked to VHL mutations.
Figures




References
-
- An, Z., P. Jiang, X. Wang, A. R. Moossa, and R. M. Hoffman. 1999. Development of a high metastatic orthotopic model of human renal cell carcinoma in nude mice: benefits of fragment implantation compared to cell-suspension injection. Clin. Exp. Metastasis 17:265-270. - PubMed
-
- Appelhoff, R. J., Y. M. Tian, R. R. Raval, H. Turley, A. L. Harris, C. W. Pugh, P. J. Ratcliffe, and J. M. Gleadle. 2004. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J. Biol. Chem. 279:38458-38465. - PubMed
-
- Aprelikova, O., G. V. Chandramouli, M. Wood, J. R. Vasselli, J. Riss, J. K. Maranchie, W. M. Linehan, and J. C. Barrett. 2004. Regulation of HIF prolyl hydroxylases by hypoxia-inducible factors. J. Cell Biochem. 92:491-501. - PubMed
-
- Bruick, R. K., and S. L. McKnight. 2001. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294:1337-1340. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
