A double-bromodomain protein, FSH-S, activates the homeotic gene ultrabithorax through a critical promoter-proximal region
- PMID: 17526731
- PMCID: PMC1952094
- DOI: 10.1128/MCB.00692-07
A double-bromodomain protein, FSH-S, activates the homeotic gene ultrabithorax through a critical promoter-proximal region
Abstract
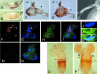
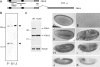
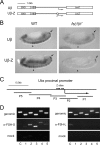
More than a dozen trithorax group (trxG) proteins are involved in activation of Drosophila HOX genes. How they act coordinately to integrate signals from distantly located enhancers is not fully understood. The female sterile (1) homeotic (fs(1)h) gene is one of the trxG genes that is most critical for Ultrabithorax (Ubx) activation. We show that one of the two double-bromodomain proteins encoded by fs(1)h acts as an essential factor in the Ubx proximal promoter. First, overexpression of the small isoform FSH-S, but not the larger one, can induce ectopic expression of HOX genes and cause body malformation. Second, FSH-S can stimulate Ubx promoter in cultured cells through a critical proximal region in a bromodomain-dependent manner. Third, purified FSH-S can bind specifically to a motif within this region that was previously known as the ZESTE site. The physiological relevance of FSH-S is ascertained using transgenic embryos containing a modified Ubx proximal promoter and chromatin immunoprecipitation. In addition, we show that FSH-S is involved in phosphorylation of itself and other regulatory factors. We suggest that FSH-S acts as a critical component of a regulatory circuitry mediating long-range effects of distant enhancers.
Figures





References
-
- Ashburner, M. 1989. Drosophila: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
-
- Beisel, C., A. Imhof, J. Greene, E. Kremmer, and F. Sauer. 2002. Histone methylation by the Drosophila epigenetic transcriptional regulator Ash1. Nature 419:857. - PubMed
-
- Biggin, M. D., S. Bickel, M. Benson, V. Pirrotta, and R. Tjian. 1988. Zeste encodes a sequence-specific transcription factor that activates the Ultrabithorax promoter in vitro. Cell 53:713-722. - PubMed
-
- Biggin, M. D., and R. Tjian. 1988. Transcription factors that activate the Ultrabithorax promoter in developmentally staged extracts. Cell 53:699-711. - PubMed
-
- Brand, A. H., and N. Perrimon. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118:401-415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
