Haploinsufficiency of Mdm2 and Mdm4 in tumorigenesis and development
- PMID: 17526734
- PMCID: PMC1952101
- DOI: 10.1128/MCB.00555-06
Haploinsufficiency of Mdm2 and Mdm4 in tumorigenesis and development
Abstract
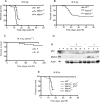
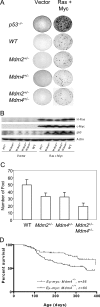
The tumor suppressor p53 is inactivated by multiple mechanisms that include mutations of the p53 gene itself and increased levels of the p53 inhibitors MDM2 and MDM4. Mice lacking Mdm2 or Mdm4 exhibit embryo-lethal phenotypes that are completely rescued by concomitant deletion of p53. Here we show that Mdm2 and Mdm4 haploinsufficiency leads to increased p53 activity, exhibited as increased sensitivity to DNA damage and decreased transformation potential. Moreover, in in vivo tumor development, Emu-myc Mdm4+/- mice show a delayed onset of B-cell lymphomas compared to Emu-myc mice. Additionally, Mdm2+/- Mdm4+/- double-heterozygous mice are not viable and exhibit defects in hematopoiesis and cerebellar development. The defects in Mdm2+/- Mdm4+/- mice are corrected by deletion of a single p53 allele. These findings highlight the exquisite sensitivity of p53 to Mdm2 and Mdm4 levels and suggest that some cell types may be more sensitive to therapeutic drugs that inhibit the Mdm-p53 interaction.
Figures



References
-
- Adams, J. M., A. W. Harris, C. A. Pinkert, L. M. Corcoran, W. S. Alexander, S. Cory, R. D. Palmiter, and R. L. Brinster. 1985. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 318:533-538. - PubMed
-
- Bond, G. L., W. Hu, E. E. Bond, H. Robins, S. G. Lutzker, N. C. Arva, J. Bargonetti, F. Barte, H. Taubert, P. Wuerl, K. Onel, L. Yip, S. J. Hwang, L. C. Strong, G. Lozano, and A. J. Levine. 2004. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell 119:591-602. - PubMed
-
- Bond, G. L., W. Hu, and A. Levine. 2005. A single nucleotide polymorphism in the MDM2 gene: from a molecular and cellular explanation to clinical effect. Cancer Res. 65:5481-5484. - PubMed
-
- Danovi, D., E. Meulmeester, D. Pasini, D. Migliorini, M. Capra, R. Frenk, P. de Graaf, S. Francoz, P. Gasparini, A. Gobbi, K. Helin, P. G. Pelicci, A. G. Jochemsen, and J.-C. Marine. 2004. Amplification of Mdmx (or Mdm4) directly contributes to tumor formation by inhibiting p53 tumor suppressor activity. Mol. Cell. Biol. 24:5835-5843. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
