Induction of nitric oxide synthase and activation of signaling proteins in Anopheles mosquitoes by the malaria pigment, hemozoin
- PMID: 17526741
- PMCID: PMC1952000
- DOI: 10.1128/IAI.00645-07
Induction of nitric oxide synthase and activation of signaling proteins in Anopheles mosquitoes by the malaria pigment, hemozoin
Abstract
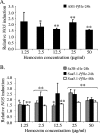
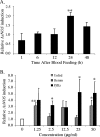
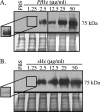
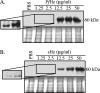
Anopheles stephensi, a major vector for malaria parasite transmission, responds to Plasmodium infection by synthesis of inflammatory levels of nitric oxide (NO), which can limit parasite development in the midgut. We have previously shown that Plasmodium falciparum glycosylphosphatidylinositols (PfGPIs) can induce A. stephensi NO synthase (AsNOS) expression in the midgut epithelium in vivo in a manner similar to the manner in which cytokines and NO are induced by PfGPIs in mammalian cells. In mosquito cells, signaling by PfGPIs and P. falciparum merozoites is mediated through Akt/protein kinase B (Akt/PKB), the mitogen-activated protein kinase kinase DSOR1, and extracellular signal-regulated kinase (ERK). In mammalian cells, a second parasite factor, malaria pigment or hemozoin (Hz), signals NOS induction through ERK- and nuclear factor kappa B-dependent pathways and has been demonstrated to be a novel proinflammatory ligand for Toll-like receptor 9. In this study, we demonstrate that Hz can also induce AsNOS gene expression in immortalized A. stephensi and Anopheles gambiae cell lines in vitro and in A. stephensi midgut tissue in vivo. In mosquito cells, Hz signaling is mediated through transforming growth factor beta-associated kinase 1, Akt/PKB, ERK, and atypical protein kinase C zeta/lambda. Our results show that Hz is a prominent parasite-derived signal for Anopheles and that signaling pathways activated by PfGPIs and Hz have both unique and shared components. Together with our previous findings, our data indicate that parasite signaling of innate immunity is conserved in mosquito and mammalian cells.
Figures


References
-
- An, H., Y. Yu, M. Zhang, H. Xu, R. Qi, X. Yan, S. Liu, W. Wang, Z. Guo, J. Guo, Z. Qin, and X. Cao. 2002. Involvement of ERK, p38 and NF-kappaB signal transduction in regulation of TLR2, TLR4 and TLR9 gene expression induced by lipopolysaccharide in mouse dendritic cells. Immunology 106:38-45. - PMC - PubMed
-
- Anstey, N. M., J. B. Weinberg, M. Y. Hassanali, E. D. Mwaikambo, D. Manyenga, M. A. Misukonis, D. R. Arnelle, D. Hollis, M. I. McDonald, and D. L. Granger. 1996. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J. Exp. Med. 184:557-567. - PMC - PubMed
-
- Arese, P., and E. Schwarzer. 1997. Malarial pigment (haemozoin): a very active ‘inert’ substance. Ann. Trop. Med. Parasitol. 91:501-516. - PubMed
-
- Berner, R., W. Rudin, and H. Hecker. 1983. Peritrophic membranes and protease activity in the midgut of the malaria mosquito, Anopheles stephensi (Liston) (Insecta: Diptera) under normal and experimental conditions. J. Ultrastruct. Res. 83:195-204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

