Accumulation of pathological prion protein PrPSc in the skin of animals with experimental and natural scrapie
- PMID: 17530923
- PMCID: PMC1876502
- DOI: 10.1371/journal.ppat.0030066
Accumulation of pathological prion protein PrPSc in the skin of animals with experimental and natural scrapie
Abstract
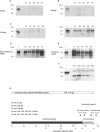
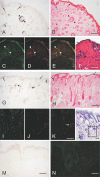
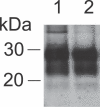
Prion infectivity and its molecular marker, the pathological prion protein PrP(Sc), accumulate in the central nervous system and often also in lymphoid tissue of animals or humans affected by transmissible spongiform encephalopathies. Recently, PrP(Sc) was found in tissues previously considered not to be invaded by prions (e.g., skeletal muscles). Here, we address the question of whether prions target the skin and show widespread PrP(Sc) deposition in this organ in hamsters perorally or parenterally challenged with scrapie. In hamsters fed with scrapie, PrP(Sc) was detected before the onset of symptoms, but the bulk of skin-associated PrP(Sc) accumulated in the clinical phase. PrP(Sc) was localized in nerve fibres within the skin but not in keratinocytes, and the deposition of PrP(Sc) in skin showed no dependence from the route of infection and lymphotropic dissemination. The data indicated a neurally mediated centrifugal spread of prions to the skin. Furthermore, in a follow-up study, we examined sheep naturally infected with scrapie and detected PrP(Sc) by Western blotting in skin samples from two out of five animals. Our findings point to the skin as a potential reservoir of prions, which should be further investigated in relation to disease transmission.
Conflict of interest statement
Figures


References
-
- Beekes M, McBride P. The spread of prions through the body in naturally acquired transmissible spongiform encephalopathies. FEBS J. 2007;274:588–605. - PubMed
-
- Beekes M, Baldauf E, Diringer H. Sequential appearance and accumulation of pathognomonic markers in the central nervous system of hamsters orally infected with scrapie. J Gen Virol. 1996;77:1925–1934. - PubMed
-
- van Keulen LJ, Vromans ME, van Zijderveld FG. Early and late pathogenesis of natural scrapie infection in sheep. APMIS. 2002;110:23–32. - PubMed
-
- Ironside JW. Pathology of variant Creutzfeldt-Jakob disease. Arch Virol Suppl. 2000. pp. 143–151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

