Directed engineering of umbilical cord blood stem cells to produce C-peptide and insulin
- PMID: 17531081
- PMCID: PMC6496474
- DOI: 10.1111/j.1365-2184.2007.00439.x
Directed engineering of umbilical cord blood stem cells to produce C-peptide and insulin
Abstract
Objectives: In this study, we investigated the potential of umbilical cord blood stem cell lineages to produce C-peptide and insulin.
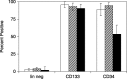
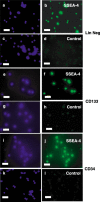
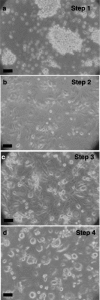
Materials and methods: Lineage negative, CD133+ and CD34+ cells were analyzed by flow cytometry to assess expression of cell division antigens. These lineages were expanded in culture and subjected to an established protocol to differentiate mouse embryonic stem cells (ESCs) toward the pancreatic phenotype. Phase contrast and fluorescence immunocytochemistry were used to characterize differentiation markers with particular emphasis on insulin and C-peptide.
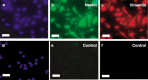
Results: All 3 lineages expressed SSEA-4, a marker previously reported to be restricted to the ESC compartment. Phase contrast microscopy showed all three lineages recapitulated the treatment-dependent morphological changes of ESCs as well as the temporally restricted expression of nestin and vimentin during differentiation. After engineering, each isolate contained both C-peptide and insulin, a result also obtained following a much shorter protocol for ESCs.
Conclusions: Since C-peptide can only be derived from de novo synthesis and processing of pre-proinsulin mRNA and protein, we conclude that these results are the first demonstration that human umbilical cord blood-derived stem cells can be engineered to engage in de novo synthesis of insulin.
Figures


References
-
- Abuljadayel IS (2003) Induction of stem cell‐like plasticity in mononuclear cells derived from unmobilised adult human peripheral blood. Curr. Med. Res. Opin. 19, 355–375. - PubMed
-
- Ali NN, Edgar AJ, Samadikuchaksaraei A, Timson CM, Romanska HM, Polak JM, Bishop AE (2002) Derivation of type II alveolar epithelial cells from murine embryonic stem cells. Tissue Eng. 8, 541–550. - PubMed
-
- Blyszczuk P, Asbrand C, Rozzo A, Kania G, St‐Onge L, Rupnik M, Wobus AM (2004) Embryonic stem cells differentiate into insulin‐producing cells without selection of nestin‐expressing cells. Int. J. Dev Biol. 48, 1095–1104. - PubMed
-
- Blyszczuk P, Wobus AM (2004) Stem cells and pancreatic differentiation in vitro . J. Biotechnol. 113, 3–13. - PubMed
-
- Bock T (2004) The source (S) for new pancreatic beta cells in adult life. Bioessays 26, 1156–1159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

