Chromophore-assisted light inactivation of pKi-67 leads to inhibition of ribosomal RNA synthesis
- PMID: 17531085
- PMCID: PMC6496591
- DOI: 10.1111/j.1365-2184.2007.00433.x
Chromophore-assisted light inactivation of pKi-67 leads to inhibition of ribosomal RNA synthesis
Abstract
Objectives: Expression of the nuclear Ki-67 protein (pKi-67) is strongly associated with cell proliferation. For this reason, antibodies against this protein are widely used as prognostic tools for the assessment of cell proliferation in biopsies from cancer patients. Despite this broad application in histopathology, functional evidence for the physiological role of pKi-67 is still missing. Recently, we proposed a function of pKi-67 in the early steps of ribosomal RNA (rRNA) synthesis. Here, we have examined the involvement of pKi-67 in this process by photochemical inhibition using chromophore-assisted light inactivation (CALI).
Materials and methods: Anti-pKi-67 antibodies were labelled with the fluorochrome fluorescein 5(6)-isothiocyanate and were irradiated after binding to their target protein.
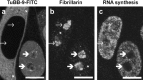
Results: Performing CALI in vitro on cell lysates led to specific cross-linking of pKi-67. Moreover, the upstream binding factor (UBF) necessary for rRNA transcription was also partly subjected to cross-link formation, indicating a close spatial proximity of UBF and pKi-67. CALI in living cells, using micro-injected antibody, caused a striking relocalization of UBF from foci within the nucleoli to spots located at the nucleolar rim or within the nucleoplasm. pKi-67-CALI resulted in dramatic inhibition of RNA polymerase I-dependent nucleolar rRNA synthesis, whereas RNA polymerase II-dependent nucleoplasmic RNA synthesis remained almost unaltered.
Conclusions: Our data presented here argue for a crucial role of pKi-67 in RNA polymerase I-dependent nucleolar rRNA synthesis.
Figures


Similar articles
-
Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells.J Cell Physiol. 2006 Mar;206(3):624-35. doi: 10.1002/jcp.20494. J Cell Physiol. 2006. PMID: 16206250
-
Nucleolar protein upstream binding factor is sequestered into adenovirus DNA replication centres during infection without affecting RNA polymerase I location or ablating rRNA synthesis.J Cell Sci. 2006 Jun 15;119(Pt 12):2621-31. doi: 10.1242/jcs.02982. J Cell Sci. 2006. PMID: 16763197
-
In vivo evidence that TATA-binding protein/SL1 colocalizes with UBF and RNA polymerase I when rRNA synthesis is either active or inactive.J Cell Biol. 1996 Apr;133(2):225-34. doi: 10.1083/jcb.133.2.225. J Cell Biol. 1996. PMID: 8609157 Free PMC article.
-
The nucleolus and transcription of ribosomal genes.Biol Cell. 2004 Oct;96(8):579-94. doi: 10.1016/j.biolcel.2004.04.015. Biol Cell. 2004. PMID: 15519693 Review.
-
[Structure and function of the nucleolus].Tanpakushitsu Kakusan Koso. 2006 Nov;51(14 Suppl):1950-6. Tanpakushitsu Kakusan Koso. 2006. PMID: 17471891 Review. Japanese. No abstract available.
Cited by
-
Correlation and significance of COX-2, Ki67, VEGF and other immune indexes with the growth of malignant pulmonary nodules.J Cardiothorac Surg. 2022 Nov 16;17(1):290. doi: 10.1186/s13019-022-02039-7. J Cardiothorac Surg. 2022. PMID: 36384712 Free PMC article.
-
Mesenchymal stem cells inhibited development of lung cancer induced by chemical carcinogens in a rat model.Am J Transl Res. 2017 Jun 15;9(6):2891-2900. eCollection 2017. Am J Transl Res. 2017. PMID: 28670377 Free PMC article.
-
Ki-67 as a molecular target for therapy in an in vitro three-dimensional model for ovarian cancer.Cancer Res. 2010 Nov 15;70(22):9234-42. doi: 10.1158/0008-5472.CAN-10-1190. Epub 2010 Nov 2. Cancer Res. 2010. PMID: 21045152 Free PMC article.
-
Analysis of human Ki-67 gene promoter and identification of the Sp1 binding sites for Ki-67 transcription.Tumour Biol. 2012 Feb;33(1):257-66. doi: 10.1007/s13277-011-0277-z. Epub 2011 Nov 29. Tumour Biol. 2012. PMID: 22125028
-
Myc controls NK cell development, IL-15-driven expansion, and translational machinery.Life Sci Alliance. 2023 Apr 27;6(7):e202302069. doi: 10.26508/lsa.202302069. Print 2023 Jul. Life Sci Alliance. 2023. PMID: 37105715 Free PMC article.
References
-
- Alexandrow MG, Ritzi M, Pemov A, Hamlin JL (2002) A potential role for mini‐chromosome maintenance (MCM) proteins in initiation at the dihydrofolate reductase replication origin. J. Biol. Chem. 277, 2702–2708. - PubMed
-
- Beck S, Sakurai T, Eustace BK, Beste G, Schier R, Rudert F, Jay DG (2002) Fluorophore‐assisted light inactivation: a high‐throughput tool for direct target validation of proteins. Proteomics 2, 247–255. - PubMed
-
- Bell SP, Learned RM, Jantzen HM, Tjian R (1988) Functional cooperativity between transcription factors UBF1 and SL1 mediates human ribosomal RNA synthesis. Science 241, 1192–1197. - PubMed
-
- Biggiogera M, Malatesta M, Abolhassani‐Dadras S, Amalric F, Rothblum LI, Fakan S (2001) Revealing the unseen: the organizer region of the nucleolus. J. Cell Sci. 114, 3199–3205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

