Neural network interactions and ingestive behavior control during anorexia
- PMID: 17531275
- PMCID: PMC2570355
- DOI: 10.1016/j.physbeh.2007.04.010
Neural network interactions and ingestive behavior control during anorexia
Abstract
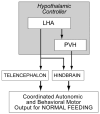
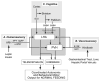
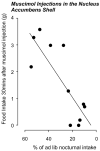
Many models have been proposed over the years to explain how motivated feeding behavior is controlled. One of the most compelling is based on the original concepts of Eliot Stellar whereby sets of interosensory and exterosensory inputs converge on a hypothalamic control network that can either stimulate or inhibit feeding. These inputs arise from information originating in the blood, the viscera, and the telencephalon. In this manner the relative strengths of the hypothalamic stimulatory and inhibitory networks at a particular time dictates how an animal feeds. Anorexia occurs when the balance within the networks consistently favors the restraint of feeding. This article discusses experimental evidence supporting a model whereby the increases in plasma osmolality that result from drinking hypertonic saline activate pathways projecting to neurons in the paraventricular nucleus of the hypothalamus (PVH) and lateral hypothalamic area (LHA). These neurons constitute the hypothalamic controller for ingestive behavior, and receive a set of afferent inputs from regions of the brain that process sensory information that is critical for different aspects of feeding. Important sets of inputs arise in the arcuate nucleus, the hindbrain, and in the telencephalon. Anorexia is generated in dehydrated animals by way of osmosensitive projections to the behavior control neurons in the PVH and LHA, rather than by actions on their afferent inputs.
Figures


Similar articles
-
The role of hypothalamic ingestive behavior controllers in generating dehydration anorexia: a Fos mapping study.Am J Physiol Regul Integr Comp Physiol. 2008 Oct;295(4):R1009-19. doi: 10.1152/ajpregu.90425.2008. Epub 2008 Jul 30. Am J Physiol Regul Integr Comp Physiol. 2008. PMID: 18667712 Free PMC article.
-
The functional architecture of dehydration-anorexia.Physiol Behav. 2010 Jul 14;100(5):472-7. doi: 10.1016/j.physbeh.2010.04.010. Epub 2010 Apr 23. Physiol Behav. 2010. PMID: 20399797 Free PMC article. Review.
-
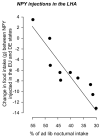
Site-specific attenuation of food intake but not the latency to eat after hypothalamic injections of neuropeptide Y in dehydrated-anorexic rats.Am J Physiol Regul Integr Comp Physiol. 2009 Dec;297(6):R1813-21. doi: 10.1152/ajpregu.00116.2009. Epub 2009 Sep 9. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19741057 Free PMC article.
-
Understanding the neural control of ingestive behaviors: helping to separate cause from effect with dehydration-associated anorexia.Horm Behav. 2000 Jun;37(4):261-83. doi: 10.1006/hbeh.2000.1581. Horm Behav. 2000. PMID: 10860672 Review.
-
Distinct patterns of neuropeptide gene expression in the lateral hypothalamic area and arcuate nucleus are associated with dehydration-induced anorexia.J Neurosci. 1999 Jul 15;19(14):6111-21. doi: 10.1523/JNEUROSCI.19-14-06111.1999. J Neurosci. 1999. PMID: 10407047 Free PMC article.
Cited by
-
The physiological control of eating: signals, neurons, and networks.Physiol Rev. 2022 Apr 1;102(2):689-813. doi: 10.1152/physrev.00028.2020. Epub 2021 Sep 6. Physiol Rev. 2022. PMID: 34486393 Free PMC article.
-
The role of hypothalamic ingestive behavior controllers in generating dehydration anorexia: a Fos mapping study.Am J Physiol Regul Integr Comp Physiol. 2008 Oct;295(4):R1009-19. doi: 10.1152/ajpregu.90425.2008. Epub 2008 Jul 30. Am J Physiol Regul Integr Comp Physiol. 2008. PMID: 18667712 Free PMC article.
-
Central, but not basolateral, amygdala is critical for control of feeding by aversive learned cues.J Neurosci. 2009 Dec 2;29(48):15205-12. doi: 10.1523/JNEUROSCI.3656-09.2009. J Neurosci. 2009. PMID: 19955373 Free PMC article.
-
Interoception, contemplative practice, and health.Front Psychol. 2015 Jun 9;6:763. doi: 10.3389/fpsyg.2015.00763. eCollection 2015. Front Psychol. 2015. PMID: 26106345 Free PMC article.
-
Critical determinants of hypothalamic appetitive neuropeptide development and expression: species considerations.Front Neuroendocrinol. 2010 Jan;31(1):16-31. doi: 10.1016/j.yfrne.2009.10.001. Epub 2009 Oct 12. Front Neuroendocrinol. 2010. PMID: 19822169 Free PMC article. Review.
References
-
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–95. - PubMed
-
- Munzberg H, Myers MG., Jr Molecular anatomical, determinants of central leptin resistance. Nat Neurosci. 2005;8:566–70. - PubMed
-
- Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. - PubMed
-
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. - PubMed
-
- Sawchenko PE. Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. J Comp Neurol. 1998;402:435–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

