Ubiquitination and proteasomal degradation of interferon regulatory factor-3 induced by Npro from a cytopathic bovine viral diarrhea virus
- PMID: 17531282
- PMCID: PMC2000802
- DOI: 10.1016/j.virol.2007.04.023
Ubiquitination and proteasomal degradation of interferon regulatory factor-3 induced by Npro from a cytopathic bovine viral diarrhea virus
Abstract
The pathogenesis of bovine viral diarrhea virus (BVDV) infections is complex and only partly understood. It remains controversial whether interferon is produced in cells infected with cytopathic(cp) BVDVs which do not persist in vivo. We show here that a cpBVDV (NADL strain) does not induce interferon responses in cell culture and blocks induction of interferon-stimulated genes by a super-infecting paramyxovirus. cpBVDV infection causes a marked loss of interferon regulatory factor 3 (IRF-3), a cellular transcription factor that controls interferon synthesis. This is attributed to expression of Npro, but not its protease activity. Npro interacts with IRF-3, prior to its activation by virus-induced phosphorylation, resulting in polyubiquitination and subsequent proteasomal degradation of IRF-3. Thermal inactivation of the E1 ubiquitin-activating enzyme prevents Npro-induced IRF-3 loss. These data suggest that inhibition of interferon production is a shared feature of both ncp and cpBVDVs and provide new insights regarding IRF-3 regulation in pestivirus pathogenesis.
Figures

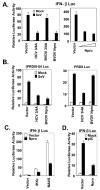
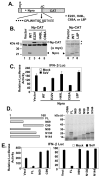


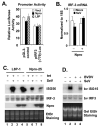
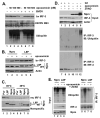
References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

