Deletion of the acid-sensing ion channel ASIC3 prevents gastritis-induced acid hyperresponsiveness of the stomach-brainstem axis
- PMID: 17531389
- PMCID: PMC4359900
- DOI: 10.1016/j.pain.2007.04.025
Deletion of the acid-sensing ion channel ASIC3 prevents gastritis-induced acid hyperresponsiveness of the stomach-brainstem axis
Abstract
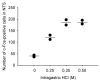
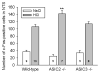
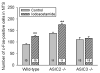
Gastric acid challenge of the rat and mouse stomach is signalled to the brainstem as revealed by expression of c-Fos. The molecular sensors relevant to the detection of gastric mucosal acidosis are not known. Since the acid-sensing ion channels ASIC2 and ASIC3 are expressed by primary afferent neurons, we examined whether knockout of the ASIC2 or ASIC3 gene modifies afferent signalling of a gastric acid insult in the normal and inflamed stomach. The stomach of conscious mice (C57BL/6) was challenged with intragastric HCl; two hours later the activation of neurons in the nucleus tractus solitarii (NTS) of the brainstem was visualized by c-Fos immunocytochemistry. Mild gastritis was induced by addition of iodoacetamide (0.1%) to the drinking water for 7 days. Exposure of the gastric mucosa to HCl (0.25M) caused a 3-fold increase in the number of c-Fos-positive neurons in the NTS. This afferent input to the NTS remained unchanged by ASIC3 knockout, whereas ASIC2 knockout augmented the c-Fos response to gastric HCl challenge by 33% (P<0.01). Pretreatment of wild-type mice with iodoacetamide induced mild gastritis, as revealed by increased myeloperoxidase activity, and enhanced the number of NTS neurons responding to gastric HCl challenge by 41% (P<0.01). This gastric acid hyperresponsiveness was absent in ASIC3 knockout mice but fully preserved in ASIC2 knockout mice. The current data indicate that ASIC3 plays a major role in the acid hyperresponsiveness associated with experimental gastritis. In contrast, ASIC2 appears to dampen acid-evoked input from the stomach to the NTS.
Figures


Similar articles
-
Increase in gastric acid-induced afferent input to the brainstem in mice with gastritis.Neuroscience. 2007 Mar 30;145(3):1108-19. doi: 10.1016/j.neuroscience.2006.12.025. Epub 2007 Feb 14. Neuroscience. 2007. PMID: 17303342 Free PMC article.
-
Afferent signalling from the acid-challenged rat stomach is inhibited and gastric acid elimination is enhanced by lafutidine.BMC Gastroenterol. 2009 Jun 2;9:40. doi: 10.1186/1471-230X-9-40. BMC Gastroenterol. 2009. PMID: 19490646 Free PMC article.
-
Endogenous neuropeptide Y depresses the afferent signaling of gastric acid challenge to the mouse brainstem via neuropeptide Y type Y2 and Y4 receptors.Neuroscience. 2005;136(4):1097-107. doi: 10.1016/j.neuroscience.2005.08.038. Epub 2005 Oct 10. Neuroscience. 2005. PMID: 16216428 Free PMC article.
-
Acid-sensing ion channels in gastrointestinal function.Neuropharmacology. 2015 Jul;94:72-9. doi: 10.1016/j.neuropharm.2014.12.009. Epub 2015 Jan 9. Neuropharmacology. 2015. PMID: 25582294 Free PMC article. Review.
-
Afferent signalling of gastric acid challenge.J Physiol Pharmacol. 2003 Dec;54 Suppl 4:43-53. J Physiol Pharmacol. 2003. PMID: 15075448 Review.
Cited by
-
Identifying the Ion Channels Responsible for Signaling Gastro-Intestinal Based Pain.Pharmaceuticals (Basel). 2010 Aug 26;3(9):2768-2798. doi: 10.3390/ph3092768. Pharmaceuticals (Basel). 2010. PMID: 27713376 Free PMC article. Review.
-
Antibody-induced pain-like behavior and bone erosion: links to subclinical inflammation, osteoclast activity, and acid-sensing ion channel 3-dependent sensitization.Pain. 2022 Aug 1;163(8):1542-1559. doi: 10.1097/j.pain.0000000000002543. Epub 2021 Nov 19. Pain. 2022. PMID: 34924556 Free PMC article.
-
Acid-Sensing Ion Channels as Potential Therapeutic Targets in Neurodegeneration and Neuroinflammation.Mediators Inflamm. 2017;2017:3728096. doi: 10.1155/2017/3728096. Epub 2017 Sep 19. Mediators Inflamm. 2017. PMID: 29056828 Free PMC article. Review.
-
Mechanisms of intragastric pH sensing.Curr Gastroenterol Rep. 2010 Dec;12(6):465-70. doi: 10.1007/s11894-010-0147-7. Curr Gastroenterol Rep. 2010. PMID: 20938760 Free PMC article. Review.
-
Marine Toxins and Nociception: Potential Therapeutic Use in the Treatment of Visceral Pain Associated with Gastrointestinal Disorders.Toxins (Basel). 2019 Jul 31;11(8):449. doi: 10.3390/toxins11080449. Toxins (Basel). 2019. PMID: 31370176 Free PMC article. Review.
References
-
- Altschuler SM, Bao XM, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283:248–268. - PubMed
-
- Boscan P, Pickering AE, Paton JF. The nucleus of the solitary tract: an integrating station for nociceptive and cardiorespiratory afferents. Exp Physiol. 2002;87:259–266. - PubMed
-
- Danzer M, Jocic M, Samberger C, Painsipp E, Bock E, Pabst MA, Crailsheim K, Schicho R, Lippe IT, Holzer P. Stomach-brain communication by vagal afferents in response to luminal acid backdiffusion, gastrin, and gastric acid secretion. Am J Physiol. 2004;286:G403–G411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

