Remyelination in multiple sclerosis
- PMID: 17531860
- PMCID: PMC7112255
- DOI: 10.1016/S0074-7742(07)79026-8
Remyelination in multiple sclerosis
Abstract
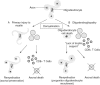
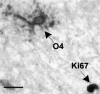
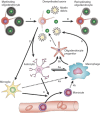
Remyelination is the phenomenon by which new myelin sheaths are generated around axons in the adult central nervous system (CNS). This follows the pathological loss of myelin in diseases like multiple sclerosis (MS). Remyelination can restore conduction properties to axons (thereby restoring neurological function) and is increasingly believed to exert a neuroprotective role on axons. Remyelination occurs in many MS lesions but becomes increasingly incomplete/inadequate and eventually fails in the majority of lesions and patients. Efforts to understand the causes for this failure of regeneration have fueled research into the biology of remyelination and the complex, interdependent cellular and molecular factors that regulate this process. Examination of the mechanisms of repair of experimental lesions has demonstrated that remyelination occurs in two major phases. The first consists of colonization of lesions by oligodendrocyte progenitor cells (OPCs), the second the differentiation of OPCs into myelinating oligodendrocytes that contact demyelinated axons to generate functional myelin sheaths. Several intracellular and extracellular molecules have been identified that mediate these two phases of repair. Theoretically, the repair of demyelinating lesions can be promoted by enhancing the intrinsic repair process (by providing one or more remyelination-enhancing factors or via immunoglobulin therapy). Alternatively, endogenous repair can be bypassed by introducing myelinogenic cells into demyelinated areas; several cellular candidates have been identified that can mediate repair of experimental demyelinating lesions. Future challenges confronting therapeutic strategies to enhance remyelination will involve the translation of findings from basic science to clinical demyelinating disease.
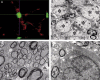
Figures


References
-
- Adams C.W.M. “A Colour Atlas of Multiple Sclerosis and Other Myelin Disorders.”. Wolfe Medical Publications; London: 1989.
-
- Antony J.M., van Marle G., Opii W., Butterfield D.A., Mallet F., Yong V.W., Wallace J.L., Deacon R.M., Warren K., Power C. Human endogenous retrovirus glycoprotein‐mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat. Neurosci. 2004;7:1088–1095. - PubMed
-
- Arnett H.A., Mason J., Marino M., Suzuki K., Matsushima G.K., Ting J.P. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat. Neurosci. 2001;4:1116–1122. - PubMed
-
- Arnett H.A., Fancy S.P., Alberta J.A., Zhao C., Plant S.R., Kaing S., Raine C.S., Rowitch D.H., Franklin R.J., Stiles C.D. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;17:2111–2115. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

