Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
- PMID: 17531949
- PMCID: PMC2039921
- DOI: 10.1016/j.abb.2007.04.014
Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria
Abstract
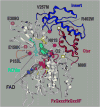
Impaired conversion of trimethylamine to trimethylamine N-oxide by human flavin containing monooxygenase 3 (FMO3) is strongly associated with primary trimethylaminuria, also known as 'fish-odor' syndrome. Numerous non-synonymous mutations in FMO3 have been identified in patients suffering from this metabolic disorder (e.g., N61S, M66I, P153L, and R492W), but the molecular mechanism(s) underlying the functional deficit attributed to these alleles has not been elucidated. The purpose of the present study was to determine the impact of these disease-associated genetic variants on FMO3 holoenzyme formation and on steady-state kinetic parameters for metabolism of several substrates, including trimethylamine. For comparative purposes, several common allelic variants not associated with primary trimethylaminuria (i.e., E158K, V257M, E308G, and the E158K/E308G haplotype) were also analyzed. When recombinantly expressed in insect cells, only the M66I and R492W mutants failed to incorporate/retain the FAD cofactor. Of the remaining mutant proteins P153L and N61S displayed substantially reduced (<10%) catalytic efficiencies for trimethylamine N-oxygenation relative to the wild-type enzyme. For N61S, reduced catalytic efficiency was solely a consequence of an increased K(m), whereas for P153L, both K(m) and k(cat) were altered. Similar results were obtained when benzydamine N-oxygenation was monitored. A homology model for FMO3 was constructed based on the crystal structure for yeast FMO which places the N61 residue alone, of the mutants analyzed here, in close proximity to the FAD catalytic center. These data demonstrate that primary trimethylaminuria is multifactorial in origin in that enzyme dysfunction can result from kinetic incompetencies as well as impaired assembly of holoprotein.
Figures


 ), P153L (▲) and N61S (●) catalyzed TMA N-oxygenation.
), P153L (▲) and N61S (●) catalyzed TMA N-oxygenation.
Similar articles
-
Genetic variants of flavin-containing monooxygenase 3 (FMO3) derived from Japanese subjects with the trimethylaminuria phenotype and whole-genome sequence data from a large Japanese database.Drug Metab Pharmacokinet. 2019 Oct;34(5):334-339. doi: 10.1016/j.dmpk.2019.06.001. Epub 2019 Jun 27. Drug Metab Pharmacokinet. 2019. PMID: 31401033
-
Novel variants in outer protein surface of flavin-containing monooxygenase 3 found in an Argentinian case with impaired capacity for trimethylamine N-oxygenation.Drug Metab Pharmacokinet. 2020 Aug;35(4):383-388. doi: 10.1016/j.dmpk.2020.05.003. Epub 2020 May 18. Drug Metab Pharmacokinet. 2020. PMID: 32653296
-
Human flavin-containing monooxygenase form 3: cDNA expression of the enzymes containing amino acid substitutions observed in individuals with trimethylaminuria.Chem Res Toxicol. 1997 Aug;10(8):837-41. doi: 10.1021/tx9700533. Chem Res Toxicol. 1997. PMID: 9282831
-
Trimethylaminuria and a human FMO3 mutation database.Hum Mutat. 2003 Sep;22(3):209-13. doi: 10.1002/humu.10252. Hum Mutat. 2003. PMID: 12938085 Review.
-
Human flavin-containing monooxygenase (form 3): polymorphisms and variations in chemical metabolism.Pharmacogenomics. 2002 May;3(3):325-39. doi: 10.1517/14622416.3.3.325. Pharmacogenomics. 2002. PMID: 12052141 Review.
Cited by
-
Association of FMO3 rs1736557 polymorphism with clopidogrel response in Chinese patients with coronary artery disease.Eur J Clin Pharmacol. 2021 Mar;77(3):359-368. doi: 10.1007/s00228-020-03024-6. Epub 2020 Oct 21. Eur J Clin Pharmacol. 2021. PMID: 33089397
-
Association of FMO3 Variants and Trimethylamine N-Oxide Concentration, Disease Progression, and Mortality in CKD Patients.PLoS One. 2016 Aug 11;11(8):e0161074. doi: 10.1371/journal.pone.0161074. eCollection 2016. PLoS One. 2016. PMID: 27513517 Free PMC article.
-
Inter-individual variation in flavin-containing monooxygenase 3 in livers from Japanese: correlation with hepatic transcription factors.Drug Metab Pharmacokinet. 2009;24(3):218-25. doi: 10.2133/dmpk.24.218. Drug Metab Pharmacokinet. 2009. PMID: 19571433 Free PMC article.
-
Impacts of the Callipyge mutation on ovine plasma metabolites and muscle fibre type.PLoS One. 2014 Jun 17;9(6):e99726. doi: 10.1371/journal.pone.0099726. eCollection 2014. PLoS One. 2014. PMID: 24937646 Free PMC article.
-
Trimethylamine-N-Oxide (TMAO) as a Rising-Star Metabolite: Implications for Human Health.Metabolites. 2025 Mar 24;15(4):220. doi: 10.3390/metabo15040220. Metabolites. 2025. PMID: 40278349 Free PMC article. Review.
References
-
- Holmes HC, Burns SP, Michelakakis H, Kordoni V, Bain MD, Chalmers RA, Rafter JE, Iles RA. Choline and L-carnitine as precursors of trimethylamine. Biochem Soc Trans. 1997;25:96S. - PubMed
-
- Tjoa S, Fennessey P. The identification of trimethylamine excess in man: quantitative analysis and biochemical origins. Anal Biochem. 1991;197:77–82. - PubMed
-
- Ruocco V, Florio M, Filioli FG, Guerrera V, Prota G. An unusual case of trimethylaminuria. Br J Dermatol. 1989;120:459–461. - PubMed
-
- Lin J, Cashman JR. Detoxication of tyramine by the flavin-containing monooxygenase: stereoselective formation of the trans oxime. Chem Res Toxicol. 1997;10:842–852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

