Cannabinoid-induced hyperphagia: correlation with inhibition of proopiomelanocortin neurons?
- PMID: 17532014
- PMCID: PMC2720321
- DOI: 10.1016/j.physbeh.2007.04.028
Cannabinoid-induced hyperphagia: correlation with inhibition of proopiomelanocortin neurons?
Abstract
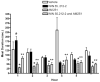
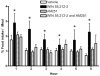
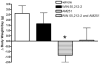
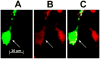
We tested the hypothesis that cannabinoids modulate feeding in male guinea pigs, and correlated cannabinoid-induced changes in feeding behavior with alterations in glutamatergic synaptic currents impinging upon proopiomelanocortin (POMC) neurons of the hypothalamic arcuate nucleus. Feeding experiments were performed as follows: after a three-day acclimation period, animals were weighed and injected with either the CB1 receptor agonist WIN 55,212-2 (1 mg/kg, s.c.), antagonist AM251 (3 mg/kg, s.c.) or their cremophore/ethanol/saline vehicle (1:1:18; 1 ml/kg, s.c.) each day for seven days. WIN 55,212-2 increased, whereas AM251 decreased, the rate of cumulative food intake. The agonist effect was manifest primarily by increases in meal frequency and the amount of food eaten per meal. By contrast, the antagonist effect was associated with decreases in meal frequency, duration and weight loss. For the electrophysiological experiments, we performed whole-cell patch-clamp recordings from POMC neurons in hypothalamic slices. WIN 55,212-2 decreased the amplitude of evoked, glutamatergic excitatory postsynaptic currents (eEPSCs) and increased the S2:S1 ratio. Conversely, AM251 increased eEPSC amplitude per se, and blocked the inhibitory effects of the agonist. WIN 55,212-2 also decreased miniature EPSC (mEPSC) frequency; whereas AM251 increased mEPSC frequency per se, and again blocked the inhibitory effect of the agonist. A subpopulation of cells exhibited an agonist-induced outward current, which was blocked by AM251, associated with increased conductance and reversed polarity near the Nernst equilibrium potential for K(+). These data demonstrate that cannabinoids regulate appetite in the guinea pig in part through both presynaptic and postsynaptic actions on anorexigenic POMC neurons.
Figures
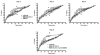
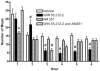




Similar articles
-
Estrogen rapidly attenuates cannabinoid-induced changes in energy homeostasis.Eur J Pharmacol. 2009 Nov 10;622(1-3):15-24. doi: 10.1016/j.ejphar.2009.09.001. Epub 2009 Sep 14. Eur J Pharmacol. 2009. PMID: 19758570 Free PMC article.
-
Estrogen differentially modulates the cannabinoid- induced presynaptic inhibition of amino acid neurotransmission in proopiomelanocortin neurons of the arcuate nucleus.Neuroendocrinology. 2006;84(2):123-37. doi: 10.1159/000096996. Epub 2006 Nov 9. Neuroendocrinology. 2006. PMID: 17106183
-
Sex differences in the cannabinoid modulation of appetite, body temperature and neurotransmission at POMC synapses.Neuroendocrinology. 2009;89(4):424-40. doi: 10.1159/000191646. Epub 2009 Jan 9. Neuroendocrinology. 2009. PMID: 19136814 Free PMC article.
-
Sex differences in the cannabinoid modulation of an A-type K+ current in neurons of the mammalian hypothalamus.J Neurophysiol. 2005 Oct;94(4):2983-6. doi: 10.1152/jn.01187.2004. Epub 2005 May 18. J Neurophysiol. 2005. PMID: 15901756
-
Sex differences in the cannabinoid regulation of energy homeostasis.Psychoneuroendocrinology. 2009 Dec;34 Suppl 1(0 1):S237-46. doi: 10.1016/j.psyneuen.2009.04.007. Psychoneuroendocrinology. 2009. PMID: 19427130 Free PMC article. Review.
Cited by
-
Steroidogenic factor 1 regulates expression of the cannabinoid receptor 1 in the ventromedial hypothalamic nucleus.Mol Endocrinol. 2008 Aug;22(8):1950-61. doi: 10.1210/me.2008-0127. Epub 2008 May 29. Mol Endocrinol. 2008. PMID: 18511494 Free PMC article.
-
Receptor subtypes and signal transduction mechanisms contributing to the estrogenic attenuation of cannabinoid-induced changes in energy homeostasis.Neuroendocrinology. 2013;97(2):160-75. doi: 10.1159/000338669. Epub 2012 Aug 28. Neuroendocrinology. 2013. PMID: 22538462 Free PMC article.
-
Hypothalamic cannabinoid signaling: Consequences for eating behavior.Pharmacol Res Perspect. 2024 Oct;12(5):e1251. doi: 10.1002/prp2.1251. Pharmacol Res Perspect. 2024. PMID: 39155548 Free PMC article. Review.
-
Sex differences in cannabinoid-regulated biology: A focus on energy homeostasis.Front Neuroendocrinol. 2016 Jan;40:101-9. doi: 10.1016/j.yfrne.2016.01.003. Epub 2016 Jan 19. Front Neuroendocrinol. 2016. PMID: 26800649 Free PMC article. Review.
-
Estrogen rapidly attenuates cannabinoid-induced changes in energy homeostasis.Eur J Pharmacol. 2009 Nov 10;622(1-3):15-24. doi: 10.1016/j.ejphar.2009.09.001. Epub 2009 Sep 14. Eur J Pharmacol. 2009. PMID: 19758570 Free PMC article.
References
-
- Williams CM, Kirkham TC. Reversal of Δ 9-THC hyperphagia by SR141716 and naloxone but not dexfenfluramine. Pharmacol. Biochem. Behav. 2002;71:341–348. - PubMed
-
- Berry EM, Mechoulam R. Tetrahydrocannabinol and endocannabinoids in feeding and appetite. Pharmac Ther. 2002;95:185–190. - PubMed
-
- Wachtel SR, Elsohly MA, Ross SA, Ambre J, De Wit H. Comparison of the subjective effects of Δ 9-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 2002;161:331–339. - PubMed
-
- Cota D, Marsicano G, Lutz B, Vicennati V, Stalla GK, Pasquali R, Pagotto U. Endogenous cannabinoid system as a modulator of food intake. Int J Obesity. 2003;27:289–301. - PubMed
-
- Fride E, Bregman T, Kirkham TC. Endocannabinoids and food intake: newborn suckling and appetite regulation in adulthood. Exp Biol Med. 2005;230:225–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

