Upregulation of hepatic angiotensin-converting enzyme 2 (ACE2) and angiotensin-(1-7) levels in experimental biliary fibrosis
- PMID: 17532087
- PMCID: PMC7114685
- DOI: 10.1016/j.jhep.2007.03.008
Upregulation of hepatic angiotensin-converting enzyme 2 (ACE2) and angiotensin-(1-7) levels in experimental biliary fibrosis
Abstract
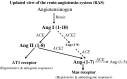
Background/aims: Angiotensin-converting enzyme 2 (ACE2), its product, angiotensin-(1-7) and its receptor, Mas, may moderate the adverse effects of angiotensin II in liver disease. We examined the expression of these novel components of the renin angiotensin system (RAS) and the production and vasoactive effects of angiotensin-(1-7) in the bile duct ligated (BDL) rat.
Methods: BDL or sham-operated rats were sacrificed at 1, 2, 3 and 4 weeks. Tissue and blood were collected for gene expression, enzyme activity and peptide measurements. In situ perfused livers were used to assess angiotensin peptide production and their effects on portal resistance.
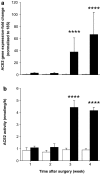
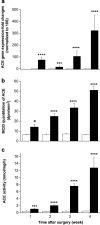
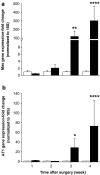
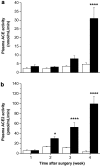
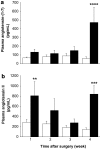
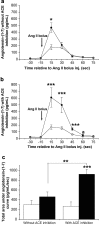
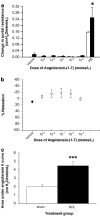
Results: Hepatic ACE2 gene and activity (P<0.0005), plasma angiotensin-(1-7) (P<0.0005) and Mas receptor expression (P<0.01) were increased following BDL compared to shams. Perfusion experiments confirmed that BDL livers produced increased angiotensin-(1-7) (P<0.05) from angiotensin II and this was augmented (P<0.01) by ACE inhibition. Whilst angiotensin II increased vasoconstriction in cirrhotic livers, angiotensin-(1-7) had no effect on portal resistance.
Conclusions: RAS activation in chronic liver injury is associated with upregulation of ACE2, Mas and hepatic conversion of angiotensin II to angiotensin-(1-7) leading to increased circulating angiotensin-(1-7). These results support the presence of an ACE2-angiotensin-(1-7)-Mas axis in liver injury which may counteract the effects of angiotensin II.
Figures
Similar articles
-
Portal pressure responses and angiotensin peptide production in rat liver are determined by relative activity of ACE and ACE2.Am J Physiol Gastrointest Liver Physiol. 2009 Jul;297(1):G98-G106. doi: 10.1152/ajpgi.00045.2009. Epub 2009 Apr 23. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19389807 Free PMC article.
-
Angiotensin-(1-7), an alternative metabolite of the renin-angiotensin system, is up-regulated in human liver disease and has antifibrotic activity in the bile-duct-ligated rat.Clin Sci (Lond). 2009 Sep 14;117(11):375-86. doi: 10.1042/CS20080647. Clin Sci (Lond). 2009. PMID: 19371232
-
Activation of the MAS receptor by angiotensin-(1-7) in the renin-angiotensin system mediates mesenteric vasodilatation in cirrhosis.Gastroenterology. 2013 Oct;145(4):874-884.e5. doi: 10.1053/j.gastro.2013.06.036. Epub 2013 Jun 22. Gastroenterology. 2013. PMID: 23796456
-
ACE2, angiotensin-(1–7), and Mas: the other side of the coin.Pflugers Arch. 2013 Jan;465(1):79-85. doi: 10.1007/s00424-012-1120-0. Pflugers Arch. 2013. PMID: 23463883 Review.
-
The Anti-Inflammatory Potential of ACE2/Angiotensin-(1-7)/Mas Receptor Axis: Evidence from Basic and Clinical Research.Curr Drug Targets. 2017;18(11):1301-1313. doi: 10.2174/1389450117666160727142401. Curr Drug Targets. 2017. PMID: 27469342 Review.
Cited by
-
Plasma levels of soluble ACE2are associated with sex, Metabolic Syndrome, and its biomarkers in a large cohort, pointing to a possible mechanism for increased severity in COVID-19.Crit Care. 2020 Jul 22;24(1):452. doi: 10.1186/s13054-020-03141-9. Crit Care. 2020. PMID: 32698840 Free PMC article. No abstract available.
-
Telmisartan attenuates hepatic fibrosis in bile duct-ligated rats.Acta Pharmacol Sin. 2012 Dec;33(12):1518-24. doi: 10.1038/aps.2012.115. Epub 2012 Oct 29. Acta Pharmacol Sin. 2012. PMID: 23103625 Free PMC article.
-
Up-regulation of components of the renin-angiotensin system in liver fibrosis in the rat induced by CCL₄.Res Vet Sci. 2013 Aug;95(1):54-8. doi: 10.1016/j.rvsc.2013.01.028. Epub 2013 Feb 21. Res Vet Sci. 2013. PMID: 23433841 Free PMC article.
-
Liver damage in the context of SARS-CoV-2. Covid-19 treatment and its effects on the liver.J Med Life. 2022 Jun;15(6):727-734. doi: 10.25122/jml-2022-0177. J Med Life. 2022. PMID: 35928369 Free PMC article. Review.
-
Activation of the Alternate Renin-Angiotensin System Correlates with the Clinical Status in Human Cirrhosis and Corrects Post Liver Transplantation.J Clin Med. 2019 Mar 27;8(4):419. doi: 10.3390/jcm8040419. J Clin Med. 2019. PMID: 30934723 Free PMC article.
References
-
- Rosenberg M.E., Smith L.J., Correa-Rotter R., Hostetter T.H. The paradox of the renin-angiotensin system in chronic renal disease. Kidney Int. 1994;45:403–410. - PubMed
-
- Johnston C.I. Tissue angiotensin converting enzyme in cardiac and vascular hypertrophy, repair, and remodeling. Hypertension. 1994;23:258–268. - PubMed
-
- Cooper M.E., Tikellis C., Thomas M.C. Preventing diabetes in patients with hypertension: one more reason to block the renin-angiotensin system. J Hypertens Suppl. 2006;24:S57–S63. - PubMed
-
- Paizis G., Cooper M.E., Schembri J.M., Tikellis C., Burrell L.M., Angus P.W. Up-regulation of components of the renin-angiotensin system in the bile duct-ligated rat liver. Gastroenterology. 2002;123:1667–1676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

