Folding amphipathic helices into membranes: amphiphilicity trumps hydrophobicity
- PMID: 17532340
- PMCID: PMC2034331
- DOI: 10.1016/j.jmb.2007.05.016
Folding amphipathic helices into membranes: amphiphilicity trumps hydrophobicity
Abstract
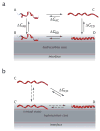
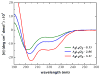
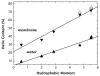
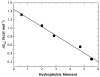
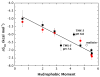
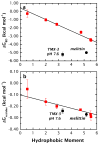
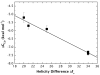
High amphiphilicity is a hallmark of interfacial helices in membrane proteins and membrane-active peptides, such as toxins and antimicrobial peptides. Although there is general agreement that amphiphilicity is important for membrane-interface binding, an unanswered question is its importance relative to simple hydrophobicity-driven partitioning. We have examined this fundamental question using measurements of the interfacial partitioning of a family of 17-residue amidated-acetylated peptides into both neutral and anionic lipid vesicles. Composed only of Ala, Leu, and Gln residues, the amino acid sequences of the peptides were varied to change peptide amphiphilicity without changing total hydrophobicity. We found that peptide helicity in water and interface increased linearly with hydrophobic moment, as did the favorable peptide partitioning free energy. This observation provides simple tools for designing amphipathic helical peptides. Finally, our results show that helical amphiphilicity is far more important for interfacial binding than simple hydrophobicity.
Figures
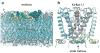
Similar articles
-
Effect of preferred binding domains on peptide retention behavior in reversed-phase chromatography: amphipathic alpha-helices.Pept Res. 1990 Jan-Feb;3(1):8-20. Pept Res. 1990. PMID: 2134049
-
Studies of the minimum hydrophobicity of alpha-helical peptides required to maintain a stable transmembrane association with phospholipid bilayer membranes.Biochemistry. 2007 Jan 30;46(4):1042-54. doi: 10.1021/bi061891b. Biochemistry. 2007. PMID: 17240988 Free PMC article.
-
Reversed-phase chromatography of synthetic amphipathic alpha-helical peptides as a model for ligand/receptor interactions. Effect of changing hydrophobic environment on the relative hydrophilicity/hydrophobicity of amino acid side-chains.J Chromatogr A. 1994 Jul 29;676(1):139-53. doi: 10.1016/0021-9673(94)00371-8. J Chromatogr A. 1994. PMID: 7921171
-
Recent advances in computational modeling of α-helical membrane-active peptides.Curr Protein Pept Sci. 2012 Nov;13(7):644-57. doi: 10.2174/138920312804142147. Curr Protein Pept Sci. 2012. PMID: 23363529 Review.
-
Is use of the hydrophobic moment a sound basis for predicting the structure-function relationships of membrane interactive alpha-helices?Curr Protein Pept Sci. 2003 Oct;4(5):357-66. doi: 10.2174/1389203033487090. Curr Protein Pept Sci. 2003. PMID: 14529529 Review.
Cited by
-
Arenavirus budding resulting from viral-protein-associated cell membrane curvature.J R Soc Interface. 2013 Jul 17;10(86):20130403. doi: 10.1098/rsif.2013.0403. Print 2013 Sep 6. J R Soc Interface. 2013. PMID: 23864502 Free PMC article.
-
Interaction of nuclease colicins with membranes: insertion depth correlates with bilayer perturbation.PLoS One. 2012;7(9):e46656. doi: 10.1371/journal.pone.0046656. Epub 2012 Sep 28. PLoS One. 2012. PMID: 23029560 Free PMC article.
-
Evolving and assembling to pierce through: Evolutionary and structural aspects of antimicrobial peptides.Comput Struct Biotechnol J. 2022 May 10;20:2247-2258. doi: 10.1016/j.csbj.2022.05.002. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35615024 Free PMC article. Review.
-
The Naturally Occurring Host Defense Peptide, LL-37, and Its Truncated Mimetics KE-18 and KR-12 Have Selected Biocidal and Antibiofilm Activities Against Candida albicans, Staphylococcus aureus, and Escherichia coli In vitro.Front Microbiol. 2017 Mar 31;8:544. doi: 10.3389/fmicb.2017.00544. eCollection 2017. Front Microbiol. 2017. PMID: 28408902 Free PMC article.
-
Membrane binding and self-association of the epsin N-terminal homology domain.J Mol Biol. 2012 Nov 9;423(5):800-17. doi: 10.1016/j.jmb.2012.08.010. Epub 2012 Aug 24. J Mol Biol. 2012. PMID: 22922484 Free PMC article.
References
-
- Eisenberg D, Weiss RM, Terwilliger TC. The helical hydrophobic moment: A measure of the amphiphilicity of a helix. Nature. 1982;299:371–374. - PubMed
-
- Rees DC, De Antonio L, Eisenberg D. Hydrophobic organization of membrane proteins. Science. 1989;245:510–513. - PubMed
-
- Granseth E, von Heijne G, Elofsson A. A study of the membrane-water interface region of membrane proteins. J Mol Biol. 2005;346:377–385. - PubMed
-
- Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300:1922–1926. - PubMed
-
- Long SB, Campbell EB, MacKinnon R. Voltage sensor of Kv1.2: Structural basis of electromechanical coupling. Science. 2005;309:903–908. - PubMed

