Implementation of high-content assay for inhibitors of mitogen-activated protein kinase phosphatases
- PMID: 17532514
- PMCID: PMC1950282
- DOI: 10.1016/j.ymeth.2007.02.006
Implementation of high-content assay for inhibitors of mitogen-activated protein kinase phosphatases
Abstract
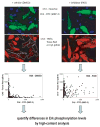
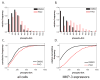
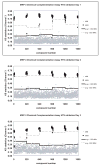
Small molecule inhibitors of protein tyrosine kinases have become both powerful chemical probes of biological processes and clinically effective therapeutics. In contrast, few small molecule inhibitors of protein tyrosine phosphatases have been identified and none are currently approved for clinical use. New cell-based high-content methods have been developed that should enable investigators to probe for selective inhibitors of diseases-relevant protein phosphatases. Details of these methods are described herein.
Figures

References
-
- Keyse SM. Curr Opin Cell Biol. 2000;12:186–192. - PubMed
-
- Farooq A, Zhou MM. Cellular Signalling. 2004;16:769–779. - PubMed
-
- Magi-Galluzzi C, Mishra R, Fiorentino M, Montironi R, Yao H, Capodieci P, Wishnow K, Kaplan I, Stork PJ, Loda M. Lab Invest. 1997;76:37–51. - PubMed
-
- Bang YJ, Kwon JH, Kang SH, Kim JW, Yang YC. Biochem Biophys Res Commun. 1998;250:43–47. - PubMed
-
- Wang H, Cheng Z, Malbon CC. Cancer Lett. 2003;191:229–237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

