Expression of the hypothalamic transcription factor Nhlh2 is dependent on energy availability
- PMID: 17532796
- PMCID: PMC3111914
- DOI: 10.1111/j.1365-2826.2007.01556.x
Expression of the hypothalamic transcription factor Nhlh2 is dependent on energy availability
Abstract
Mice with a deletion of the hypothalamic basic helix-loop-helix transcription factor Nhlh2 display adult onset obesity, implicating Nhlh2 in the neuronal circuits regulating energy availability. Nhlh2 colocalises with the hypothalamic thyrotrophin-releasing hormone (TRH) neurones in the paraventricular nucleus (PVN) and pro-opiomelanocortin (POMC) neurones in the arcuate nucleus. We show that Nhlh2 expression is significantly reduced in response to 24-h food deprivation in the arcuate nucleus, PVN, lateral hypothalamus, ventromedial hypothalamus (VMH) and dorsomedial hypothalamus (DMH). Food intake for 2 h following deprivation stimulates Nhlh2 expression in the arcuate nucleus and the PVN, and leptin injection following deprivation results in increased Nhlh2 expression in the arcuate nucleus, PVN, lateral hypothalamus, VMH, and DMH. Hypothalamic Nhlh2 expression in response to leptin injection is maximal by 2 h. Following leptin injection, Nhlh2 mRNA colocalises in POMC neurones in the arcuate nucleus and TRH neurones in the PVN. Nhlh2 mRNA expression in POMC neurones in the arcuate nucleus and TRH neurones in the PVN is reduced with energy deprivation and is stimulated with food intake and leptin injection. Modulation of POMC expression in response to changes in energy availability is not affected in mice with a targeted deletion of Nhlh2. However, deletion of Nhlh2 does result in loss of normal TRH mRNA expression in mice exposed to food deprivation and leptin stimulation. These data implicate Nhlh2 as a regulatory target of the leptin-mediated energy availability network of the hypothalamus, and TRH as a putative downstream target of Nhlh2.
Figures


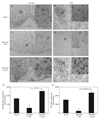
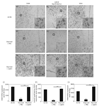



Similar articles
-
Deletion of the Nhlh2 transcription factor decreases the levels of the anorexigenic peptides alpha melanocyte-stimulating hormone and thyrotropin-releasing hormone and implicates prohormone convertases I and II in obesity.Endocrinology. 2004 Apr;145(4):1503-13. doi: 10.1210/en.2003-0834. Epub 2003 Dec 30. Endocrinology. 2004. PMID: 14701669
-
Hypothyroidism Induces Hypophagia Associated with Alterations in Protein Expression of Neuropeptide Y and Proopiomelanocortin in the Arcuate Nucleus, Independently of Hypothalamic Nuclei-Specific Changes in Leptin Signaling.Thyroid. 2016 Jan;26(1):134-43. doi: 10.1089/thy.2015.0384. Epub 2015 Dec 1. Thyroid. 2016. PMID: 26538454
-
Hypothalamic bHLH transcription factors are novel candidates in the regulation of energy balance.Eur J Neurosci. 2002 Feb;15(4):644-50. doi: 10.1046/j.1460-9568.2002.01894.x. Eur J Neurosci. 2002. PMID: 11886445
-
Energy balance pathways converging on the Nhlh2 transcription factor.Front Biosci. 2007 May 1;12:3983-93. doi: 10.2741/2365. Front Biosci. 2007. PMID: 17485352 Review.
-
The TRH neuron: a hypothalamic integrator of energy metabolism.Prog Brain Res. 2006;153:209-35. doi: 10.1016/S0079-6123(06)53012-2. Prog Brain Res. 2006. PMID: 16876577 Review.
Cited by
-
Loss of NSCL-2 in gonadotropin releasing hormone neurons leads to reduction of pro-opiomelanocortin neurons in specific hypothalamic nuclei and causes visceral obesity.J Neurosci. 2013 Jun 19;33(25):10459-70. doi: 10.1523/JNEUROSCI.5287-12.2013. J Neurosci. 2013. PMID: 23785158 Free PMC article.
-
NHLH2: at the intersection of obesity and fertility.Trends Endocrinol Metab. 2013 Aug;24(8):385-90. doi: 10.1016/j.tem.2013.04.003. Epub 2013 May 17. Trends Endocrinol Metab. 2013. PMID: 23684566 Free PMC article.
-
In Silico Examination of Single Nucleotide Missense Mutations in NHLH2, a Gene Linked to Infertility and Obesity.Int J Mol Sci. 2023 Feb 6;24(4):3193. doi: 10.3390/ijms24043193. Int J Mol Sci. 2023. PMID: 36834605 Free PMC article.
-
Nescient helix-loop-helix 2 interacts with signal transducer and activator of transcription 3 to regulate transcription of prohormone convertase 1/3.Mol Endocrinol. 2008 Jun;22(6):1438-48. doi: 10.1210/me.2008-0010. Epub 2008 Mar 20. Mol Endocrinol. 2008. PMID: 18356286 Free PMC article.
-
Leptin signaling regulates hypothalamic expression of nescient helix-loop-helix 2 (Nhlh2) through signal transducer and activator 3 (Stat3).Mol Cell Endocrinol. 2014 Mar 25;384(1-2):134-42. doi: 10.1016/j.mce.2014.01.017. Epub 2014 Jan 29. Mol Cell Endocrinol. 2014. PMID: 24486192 Free PMC article.
References
-
- Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol. 2005;493:63–71. - PubMed
-
- Good DJ. How tight are your genes? Transcriptional and posttranscriptional regulation of the leptin receptor, NPY, and POMC genes. Horm Behav. 2000;37:284–298. - PubMed
-
- Jing E, Nillni EA, Sanchez VC, Stuart RC, Good DJ. Deletion of the Nhlh2 transcription factor decreases the levels of the anorexigenic peptides alpha melanocyte-stimulating hormone and thyrotropin-releasing hormone and implicates prohormone convertases I and II in obesity. Endocrinology. 2004;145:1503–1513. - PubMed
-
- Good DJ, Porter FD, Mahon KA, Parlow AF, Westphal H, Kirsch IR. Hypogonadism and obesity in mice with a targeted deletion of the Nhlh2 gene. Nat Genet. 1997;15:397–401. - PubMed
-
- Coyle CA, Jing E, Hosmer T, Powers JB, Wade G, Good DJ. Reduced voluntary activity precedes adult-onset obesity in Nhlh2 knockout mice. Physiol Behav. 2002;77:387–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

