Cellular and humoral mechanisms of osteoclast formation in Ewing's sarcoma
- PMID: 17533390
- PMCID: PMC2359921
- DOI: 10.1038/sj.bjc.6603774
Cellular and humoral mechanisms of osteoclast formation in Ewing's sarcoma
Abstract
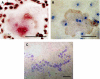
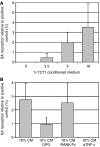
Cellular mechanisms that account for tumour osteolysis associated with Ewing's sarcoma are uncertain. Osteoclasts are marrow-derived multinucleated cells (MNCs) that effect tumour osteolysis. Osteoclasts are known to form from macrophages by both receptor activator for nuclear factor-kappaB (RANK) ligand (RANKL)-dependent and -independent mechanisms. In this study, our aim has been to determine whether tumour-associated macrophages (TAMs) isolated from Ewing's sarcoma are capable of differentiating into osteoclasts and to characterise the cellular and humoral mechanisms whereby this occurs. Tumour-associated macrophages were isolated from two Ewing's sarcomas and cultured on both coverslips and dentine slices for up to 21 days with soluble RANKL and macrophage colony stimulating factor (M-CSF). Osteoclast formation from TAMs (CD14+) was evidenced by the formation of tartrate-resistant acid phosphatase (TRAP) and vitronectin receptor (VNR)-positive MNCs, which were capable of carrying out lacunar resorption. This osteoclast formation was inhibited by the addition of bisphosphonates. Both Ewing's sarcoma-derived fibroblasts and some bone stromal cells expressed RANKL and supported osteoclast formation by a contact-dependent mechanism. We also found that osteoclast differentiation occurred when Ewing's TAMs were cultured with tumour necrosis factor (TNF)-alpha in the presence of M-CSF and that TC71 Ewing's sarcoma cells stimulated osteoclast formation through the release of a soluble factor, the action of which was abolished by an antibody to TNF-alpha. These results indicate that TAMs in Ewing's sarcoma are capable of osteoclast differentiation by both RANKL-dependent and TNF-alpha-dependent mechanisms and that Ewing's sarcoma cells produce osteoclastogenic factor(s). Our findings suggest that anti-resorptive and anti-osteoclastogenic therapies may be useful in inhibiting the osteolysis of Ewing's sarcoma.
Figures


References
-
- Adamopoulos I, Edwards J, Ferguson D, Wordsworth P, Carr A, Athanasou NA (2006) Synovial fluid macrophages are capable of osteoclast formation and resorption. J Pathol 208: 35–43 - PubMed
-
- Athanasou NA (1996) The cellular biology of bone-resorbing cells (Review). J Bone Joint Surg [A] 78A: 1096–1112 - PubMed
-
- Bugelski PJ, Corwin SP, North SM, Kirsh RL, Nicolson GL, Poste G (1987) Macrophage content of spontaneous metastases at different stages of growth. Cancer Res 47: 4141–4145 - PubMed
-
- Clohisy DR, Ogilvie CM, Carpenter RJ, Ramnaraine MLR (1996) Localised tumor-associated osteolysis involves the recruitment and activation of osteoclasts. J Orthop Res 14: 2–6 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

