Achieving signalling selectivity of ligands for the corticotropin-releasing factor type 1 receptor by modifying the agonist's signalling domain
- PMID: 17533422
- PMCID: PMC2014118
- DOI: 10.1038/sj.bjp.0707293
Achieving signalling selectivity of ligands for the corticotropin-releasing factor type 1 receptor by modifying the agonist's signalling domain
Abstract
Background and purpose: Most of the pharmaceuticals target G-protein-coupled receptors (GPCRs) which can generally activate different signalling events. The aim of this study was to achieve functional selectivity of corticotropin-releasing factor receptor type 1 (CRF(1)) ligands.
Experimental approach: We systematically substituted urocortin, a natural peptide agonist of CRF(1), with bulky amino acids (benzoyl-phenylalanine, naphthylalanine) and determined the effect of the analogues on coupling of CRF(1) to Gs- and Gi-protein in human embryonic kidney cells, using receptor binding, [(35)S]-GTPgammaS binding stimulation, and cAMP accumulation assays.
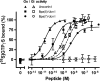
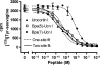
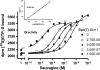
Key results: Native ligands stimulated Gs and Gi activation through CRF(1), resulting in stimulation and then inhibition of cAMP accumulation. Single replacements in urocortin at positions 6-15 led, dependent on the position and nature of the substituent, to ligands that conserved Gs activity, but were devoid of Gi activity, only stimulating cAMP accumulation, and competitively antagonized the Gi activation by sauvagine. In contrast, analogues with substitutions outside this sequence non-selectively activated Gs and Gi, as urocortin did.
Conclusions and implications: Modifications in a specific region, which we have called the signalling domain, in the polypeptide agonist urocortin resulted in analogues that behaved as agonists and, at the same time, antagonists for the activation of different G-proteins by CRF(1). This finding implies significant differences between active conformations of the receptor when coupled to different G-proteins. A similar structural encoding of signalling information in other polypeptide hormone receptor ligands would result in a general concept for the development of signalling-selective drug candidates.
Figures

References
-
- Beyermann M, Rothemund S, Heinrich N, Fechner K, Furkert J, Dathe M, et al. A role for a helical connector between two receptor binding sites of a long-chain peptide hormone. J Biol Chem. 2000;275:5702–5709. - PubMed
-
- Coy DH, Murphy WA, Sueirasdiaz J, Coy EJ, Lance VA. Structure activity studies on the N-terminal region of growth-hormone releasing-factor. J Med Chem. 1985;28:181–185. - PubMed
-
- Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–77. - PubMed
-
- Davey J. G-protein-coupled receptors: new approaches to maximise the impact of GPCRs in drug discovery – 19–20 January 2004, London, UK. Expert Opin Ther Targets. 2004;8:165–170. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

